Water Notes 8th Science Lesson 18 Notes in English
8th Science Lesson 18 Notes in English
18] Water
Introduction:
நீர்இன்று அமையாது உலெகனின் யார்யார்க்கும்
வான்இன்று அமையாது ஒழுக்கு – குறள்
Thirukkural says, without water there would be no life on the earth. Just like other living organisms, we also need water to survive. We need water for so many activities like cooking, washing, cleaning and irrigation. Water resources are getting depleted nowadays because of growing demand from increasing populations and lifestyle changes. There is also a reduction in the supply of water due to pollution of water sources and climate change which contributes to the rising variability in rainfall. We all depend on water for our living and so every individual is responsible for saving water. In this lesson, we will learn about the sources, properties and uses of water and also about water pollution and water treatment methods.
Composition:
Three fourths of our planet earth is filled with water. Water exists in three states namely solid, liquid and gas. Water on the surface of the earth is found mainly in oceans (97.25%), polar ice caps and glaciers (2.05%) and the remaining is in lakes, rivers and aquifers – ground water. Even our body is made up of water (65%) but it is not apparent. Water is a chemically stable compound. Its chemical name is di-hydrogen monoxide (H2O). It can be broken up into hydrogen (H2) and oxygen (O2) when an electrical current is passed through it. The process of breaking down of water molecules by the passage of electric current is known as electrolysis of water.
Electrolysis of Water:
Electrolysis of water can be easily demonstrated with the help of an experiment. In this experimental set up, a glass beaker fixed with two carbon electrodes is filled with water up to one third of its volume. The positive carbon electrode acts as anode and the negative carbon electrode acts as cathode. Two test tubes are placed on the electrodes as shown.
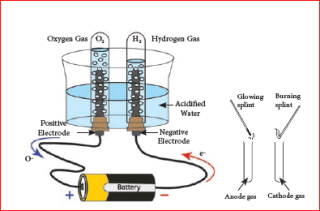
The electrodes are connected to a battery and current is passed until the test tubes are filled with a particular gas. If the gas collected is tested using a burning splint we can notice that the gas in cathode side burns with a popping sound when the extinguish splint is brought near the mouth of the test tube. This property
is usually shown by hydrogen gas and so it is confirmed that the gas inside the test tube is hydrogen. The burning splint placed near the anode side burns more brightly confirming that it is oxygen gas. This experiment shows that water is made up of hydrogen and oxygen. The ratio of hydrogen and oxygen is 2:1. Hence, for every two volumes of hydrogen collected at the cathode, there is one volume of oxygen collected at the anode.
![]()
Preparation of Water:
Water was first prepared in 1781 by an English scientist Henry Cavendish. He discovered hydrogen gas when active metals reacted with sulphuric acid. The hydrogen gas released was highly inflammable and burnt to form a colourless product called water.
Zn + H2SO4 🡪 ZnSO4 + H2↑
2H2 + O2 🡪 2H2O
Water is also produced by the reduction of metal oxide by hydrogen, burning of hydrogen in air and burning of hydrocarbons in air. Respiration of plants and animals also releases water.
C6H12O6 + 6O2 🡪 6CO2 + 6H2O + Energy
Glucose Oxygen Carbon dioxide Water
Laboratory preparation of water:
The apparatus used for the preparation of water in the laboratories is as shown. In this method, pure hydrogen gas is passed through anhydrous calcium chloride to absorb water vapour, if present. Dry hydrogen coming out of the opening is burnt with sufficient supply of air. The burnt hydrogen gas forms droplets of water, when it comes in contact with the cold flask. Distilled water without any dissolved matter is obtained by this method.

Properties of Water:
Water has some important properties which are familiar to us. But these properties are unique to water. Some of the physical and chemical properties are explained below.
Physical properties:
- Nature:
Pure water is a clear and transparent liquid. It is colourless, odourless and tasteless.
- Boiling point:
The boiling point of water is 100°C at one atmospheric pressure (1 atm). At this temperature, water boils and changes into steam. The boiling point of water increases with increase in pressure. For example, when a pressure cooker is heated, a high pressure is built inside it. The high pressure increases the boiling point of water. Thus, water remains a liquid at a higher temperature (> 100°C) in the cooker. This cooks the food faster.
- Freezing point:
Water freezes at 0°C and forms ice. Thus, the freezing point of water is 0°C. The freezing point of water decreases with increase in pressure.
- Density:
When ice cubes are put in a glass of water at room temperature, they float on the surface of the water. This is because ice water at room temperature, they float on the surface of the water. This is because ice is lighter than water. It means that the density of ice is lower than that of water. When the winter temperature is below 0°C, the water in the lake will start freezing. The frozen ice will float at the top and cover the lake. Since ice is a bad conductor of heat it does not allow heat to pass through it. So, the water below the ice remains in liquid form, where most of the aquatic life lives. This enables the aquatic animals and plants to survive even in extreme cold conditions. Density of water at different temperature is given in table.
Density of water at different temperature
| Temperature | Density |
| 0°C | 0.91 g/cc (ice) |
| 0°C | 0.97 g/cc (water) |
| 4°C | 1 g/cc |
| > 4°C | < 1 g/cc |
* 1CC = 1 cm3 = 1mL
Ice floating on water
- Anomalous expansion of water:
For the same mass of ice and of water, the volume of ice is more than that of water. It is an unusual physical property of water. In the Himalayas the temperature can go down even below 0°C. The water in the water pipes will freeze at this temperature to ice. If the pipes are not strong they can crack, develop leaks or even burst. This is because freezing of water will cause an expansion in the volume.
- Latent heat of fusion of ice:
Take some ice cubes in a beaker and place a therometer in it. Now heat the beaker. The therometer will not register any rise in temperature till all the ice melts. The question arises where does the heat energy go if there is no rise in temperature. The heat energy is utilised in changing the state of ice from solid to liquid. The amount of heat energy required by ice to change into water is called latent heat of fusion of ice. Ice has the highest latent heat of fusion, i.e., 80 calories/g. or 336 J/g.
- Latent heat of vaporization of water:
When water attains the temperature of 100°C, it starts changing its state from liquid to gaseous state. However, the temperature of water does not rise above 100°C. It is because the supplied heat energy only changes the state of the boiling water. This heat energy is stored in steam and is commonly called latent heat
of vaporization of steam. The steam has the highest latent heat of vaporization and its value is 540 calories/g or 2268 J/g.
- Specific heat capacity:
The amount of heat that is needed to raise the temperature of a unit mass of a substance by 1°C is called specific heat capacity of that substance. The specific heat capacity of water is very high. One gram of water requires 1 calorie of heat to raise its temperature by 1°C. Due to its high specific heat capacity, water takes time to become hot as well as to cool down. Thus, water can absorb a lot of heat and retain it for a longer time. This property of water is used to cool engines. Water is circulated around car engine using the radiator pump and the heat is absorbed. Thus the engine is protected from getting too hot.
Water as coolant in car engines
Chemical properties:
- Action towards litmus paper:
Pure water is neutral and it shows no action towards litmus paper.
- Stability:
Water is a very stable compound. It does not decompose into elements, when heated to ordinary temperatures. However, if it is heated to 200°C, 0.02% of water decomposes to form hydrogen and oxygen gas.
![]()
- Catalytic nature:
Water acts as a catalyst in a number of reactions. Perfectly dry hydrogen and chlorine gases do not react in the presence of sunlight. However in the presence of traces of water, the reaction takes place with explosion to produce hydrogen chloride.
![]()
- Reaction with metals:
Water reacts with some metals. Metals such as sodium, potassium and calcium react vigorously with water at room temperature. Sodium reacts with water to form hydrogen gas and sodium hydroxide solution. Due to the heat evolved in this reaction the hydrogen gas catches fire and burns.
2Na + 2H2O 🡪 2NaOH + H2
Magnesium is little more sluggish. It reacts with hot water and gives hydrogen and magnesium hydroxide solution.
Mg + 2H2O 🡪 Mg(OH)2 + H2↑
Many other metals react with water to form oxides and hydroxides. Iron is one such metal which forms iron oxide, called rust. Iron is used in many buildings, factories, bridges, ships and vehicles. The slow and gradual rusting of iron is called corrosion.
- Reaction with non-metals:
Red hot carbon (coke) reacts with steam to produce water gas (Carbon monoxide + H2).
![]()
Chlorine gas dissolves in water and produces hydrochloric acid.
![]()
Water – A Universal Solvent:
A solvent is a substance which dissolves other substances (solute). For example, in a salt solution, water is the solvent and salt is the solute. Water has a unique property to dissolve more substances than any other solvents. It can dissolve solids such as salt and sugar, liquids such as honey and milk and gases such as oxygen and carbon dioxide in it. Therefore, it is called as universal solvent.
You can see a number of concentric rings of solid matter deposited on the watch glass. These are the dissolved solids left behind after the evaporation of water. Salts, minerals and impurities are the solids dissolved in water. Dissolved salts are important for the following reasons.
- They are essential for the growth and development of plants.
- They add taste to water.
- They supply the essential minerals needed for our bodies.
- Most of the chemical reactions important for our living take place in the cells of our body with the help of water.
Apart from solids and minerals, air is also dissolved in water. Air is present in dissolved state in all natural sources of water. The solubility of oxygen in water is higher than the solubility of nitrogen. Air dissolved in water contains approximately 35.6% oxygen along with nitrogen and carbon dioxide. Air being dissolved in water is important for the following reasons.
- Air dissolved in water is important for the living organisms to survive.
- Fish extracts the oxygen from the water and expels water through the gills. Fish can survive in water only through the dissolved oxygen present in water.
- Aquatic plants make use of dissolved carbon dioxide for photosynthesis
- Carbon dioxide dissolved in water reacts with limestone to form calcium bicarbonate. Marine organisms such as snails, oysters, etc., extract calcium carbonate from calcium bicarbonate to build their shells.

Aquatic organisms
Potable Water:
Imagine you are swimming in the sea and by accident you swallow some sea water. How would you feel? You would probably feel like vomiting! The sensation of feeling nauseous is because of a lot of salt in the water. Every litre of sea water contains 35 grams of dissolved salts most commonly known as sodium chloride (NaCl). Such water is called saline water. It is not suitable for drinking and is said to be non-potable water.
The water suitable for drinking is called potable water. Every litre of potable water contains 1- 2 grams of dissolved salts, mainly common salt. In addition to the common salt, there are small amounts of calcium (Ca), magnesium (Mg), potassium (K), copper (Cu) and zinc (Zn). The minerals in water give it a certain taste. In addition, these minerals are useful for our body’s metabolism. Potable water also contains dissolved gases.
Characteristics of Potable Water:
The following are the characteristics of potable water.
- Potable water should be colourless and odourless.
- It should be transparent.
- It should be free from harmful microorganisms such as bacteria, virus and protozoa.
- It should be free from impurities such as suspended solids.
- It should contain some minerals and salts, necessary for our body and some dissolved gases to add taste.
Purification of Water:
Out of the total fresh water available on the earth, only 1% is present in water bodies such as rivers and lakes and the rest is frozen in glaciers and polar-regions. Water from these water bodies is unfit for drinking, cooking, washing or bathing because it contains suspended and dissolved impurities. It also contains micro-organisms such as bacteria. If this water is consumed without purifying, it can cause water-borne diseases such as typhoid and cholera. Therefore, water should be treated and purified before it reaches our home. In conventional water treatment plants (Figure 13.6), water is subjected to different processes for purification. These processes are discussed here.
Sedimentation:
Water from lakes or rivers is collected in large sedimentation tanks. There, it is allowed to stand undisturbed so that suspended impurities settle down at the bottom of the tank. Sometimes, a chemical substance such as potash alum is added to water, to speed up the process of sedimentation. This process is called loading. The particles of potash alum combine with the suspended impurities and make them settle down at a faster rate.
Filtration:
Water from the sedimentation tanks is then, pumped to the filtration tanks. Filtration tanks contain filter beds made up of gravel, sand, pebbles, activated charcoal and concrete. Water passes through these layers and becomes free from any remaining dissolved or suspended impurities completely.
Sterilisation:
The filtered water is treated chemically to remove the remaining germs or bacteria. This process is called sterilisation. The chemicals that are used in this process are chlorine and ozone. The water from filtration tanks is pumped into chlorination tanks, where chlorine is added to remove harmful bacteria and other germs. The process of adding chlorine, in adequate amounts, to water is called chlorination. Ozonisation is a process in which water is treated with ozone gas to kill the germs present in it.
The sterilisation of water can also be done by exposing it to air and sunlight. Oxygen from the air and sunlight destroy the germs present in water. Aeration is the process in which air under pressure is blown into filtered water. This also helps to kill the germs.

Water treatment stages
Hardness of Water:
We use soaps and detergents to wash clothes. They form lather with water that quickens the process of removal of dirt from the clothes. Water contains a number of dissolved salts and minerals. When these salts are present in very small quantities in water, it is called soft water. In this water, soaps or detergents form lather easily.

Hard water and soft water forming foam
Sometimes, minerals and salts are present in water in such a large quantity that soaps or detergents form a thick precipitate called scum instead of forming lather. This makes the removal of dirt further difficult. Such water is called hard water. Hardness of water is due to the presence of dissolved salts of calcium and magnesium. Hardness may be temporary or permanent. Temporary hardness is due to the presence of carbonate and bicarbonate salts of calcium and magnesium, and permanent hardness results due to the presence of chloride and sulphate salts of calcium and magnesium.
- Disadvantages of hard water:
- It is not good for washing clothes. It forms scum with soap and detergents, which makes the soap ineffective and also spoils the clothes further.
- It damages the utensils and containers in which it is stored and forms a hard layer.
- It forms scales on the machine parts used in industries and decreases their efficiency.
- It results in stomach ailments if consumed for a long period.
Scales on the machine pipes
- Removal of hardness:
Different methods are followed to remove the hardness from water depending on whether it is temporary hardness or permanent hardness. Some of them are explained below.
Boiling:
Temporary hardness is easily removed from water by boiling. When heated, the calcium hydrogen carbonate decomposes producing insoluble calcium carbonate. The insoluble carbonates are then filtered and removed from water. This makes the hard water soft and fit for use.
Adding washing soda:
Washing soda is used to remove permanent hardness of water. Adding washing soda converts chlorides and sulphates into insoluble carbonates. These insoluble carbonates are removed by filtration.
Ion – exchange:
Another method used to remove the hardness of water is to pass it through a column of ion-exchange resins where calcium and magnesium ions get replaced by sodium ions. This converts hard water into soft water.
Distillation:
Temporary and permanent hardness both can be removed by the method of distillation. The water obtained after distillation is called distilled water. It is the purest form of water.
Water Pollution:
Contamination of water bodies as a result of human activities is known as water pollution. Contamination of water bodies occur when harmful substances such as chemicals, sewage and waste are released into them. It produces physical, chemical and biological change in the quality of water. It degrades the water quality and renders it toxic to living organisms. Drinking polluted water has serious negative effects on human health.
Polluted water body
Water Resource in Tamil Nadu:
Fresh water resources are the sources of water that are useful to society for domestic, agricultural or industrial uses. These include surface and groundwater. Examples of surface water include rivers, reservoirs, lakes and tanks. There are 17 major river basins in Tamil Nadu with 61 reservoirs and approximately 41,948 tanks. Lakes and tanks are traditionally used in Tamil Nadu to collect rainfall during the monsoon which can be used throughout the year. Groundwater sources are called aquifers. Aquifers are layers below the ground made of coarse sand and gravel that contain spaces allowing rainwater collection. The use of groundwater is possible through open wells and bore wells.
Sources of water pollution:
When you look around you can see polluted water bodies in your surroundings. You can see lot of unwanted and harmful substances such as waste and sewage thrown into them. These substances are called pollutants. These pollutants are released by various activities from different sources. In general, sources of water pollution are classified as natural sources and man-made sources. Some of the sources of water pollution are explained below.
- Household detergents:
Household and cleaning detergents are a major cause of water pollution. Synthetic (nonbiodegradable) detergents have chemicals that do not break down and can end up polluting both surface and groundwater. Excessive use of detergents adversely affects fish and other organisms. Some shampoo, face wash, shower gel and toothpaste have small round pieces of plastic added to them. These are called microbeads. They are added for different reasons like scrubing and cleaning your skin, polishing your teeth etc. When we use products with
microbeads, they go down our drain and pollute water bodies. Fish and other animals feed them by accident and get sick.
- Domestic Sewage:
Wastewater that is disposed of from households is known as domestic sewage. Domestic sewage should be treated before being disposed of into water bodies like river, lake, etc. Untreated sewage contains impurities such as organic matter from food waste, toxic chemicals from household products and it may also contain disease-causing microbes.

Domestic water consumption
- Domestic waste and plastics:
Solid waste including plastics are disposed of or end up in water bodies such as lakes, rivers and the ocean. Plastics block drains spreading vector borne diseases such as malaria and dengue. Waste in water bodies, negatively impact aquatic life.
Plastics in domestic wastes
- Agricultural activities:
Fertilizers, pesticides and insecticides used in agriculture can dissolve in rainwater and flow into water bodies such as rivers and lakes. This causes an excess of nutrients such as nitrates and phosphates as well as toxic chemicals in water bodies. It is called Eutrophication. These sunstances can also be harmful to aquatic life.
Agricultural waste
- Industrial waste:
Many industries release toxic wastes such as lead, mercury, cyanides, cadmium, etc. If this waste is unregulated and is released into water bodies it has huge impact on humans, plants, animals and aquatic life.
Waste water from industries
- Oil spills:
There are large crude oil and natural gas reserves below the sea bed. With the increasing exploration of crude oil in the oceans, accidents in drilling and transporting oil have also increased. Oil spills cause water pollution which is harmful to aquatic life. The oil which remains floating on the water surface blocks sunshine, reduces the dissolved oxygen in water and suffocates marine organisms.
Oil spills
- Thermal pollution:
Large amount of water is used for cooling purposes in thermal and nuclear power plants and many industries. Water used for cooling purposes is discharged back to a river or to original water source at a raised temperature and sometimes with chemicals. This causes rise in temperature and decreases the amount of oxygen dissolved in water, which adversely affects the aquatic life.
Common pollutants:
Pollutants are generally classified as domestic pollutants, agricultural pollutants and industrial pollutants. The sources and effects of various water pollutants are shown below:
Types of Pollutants
| Pollutants | Sources | Effects |
| Domestic | ||
| Sodium sulphates and phosphates | Detergents | In humans they cause developmental, reproductive and neuro toxicity and endocrine disruption. Phosphates make bacteria and algae grow faster, and use up all the dissolved oxygen. This leads to a decrease in animal and plant diversity. |
| Plastic fibres and microbeads | Plastic clothing and hair, beauty and skin products | These end up in water bodies such as lakes, rivers and the ocean. Here they attract toxic chemicals. Marine animals often eat them as they confuse them as their natural source of food and the toxins can move up the food chain. |
| Agriculture | ||
| DDT (Dichloro Diphenyl Trichloro ethane) | Insecticides | If affects the central nervous system of insects, animals and humans. It accumulates in the food chain and impacts the top predators the most. |
| Nitrates and phosphates | Fertilisers | Bacteria and algae grow faster and they use up all the dissolved oxygen and this leads to a decrease in animal and plant diversity. |
| Industrial | ||
| Lead, Mercury, Cadmium, Chromium and Arsenic | Chemical, textile and leather industries and leachate from open dumping of solid waste | Toxic to animals, plants and bacteria in the water. Pollutes potable ground water. Negatively impacts human health. |
Controlling Water Pollution:
Water is precious and it is essential for all living organisms. But today almost every water body is polluted with waste ranging from plastics to toxic substances. All of us can take immediate steps to save our precious water bodies from pollution. Some simple ideas to avoid water pollution are given below:
- Use detergents that are biodegradable and avoid those that contain toxic chemicals.
- Wear clothing that is made from natural fibres such as cotton and avoid wearing synthetic fibres such as nylon, polyester etc.
- Do not throw waste such as plastics into water bodies. Always separate your waste into recyclable, non-recyclable and biodegradable so that it does not cause pollution.
- Domestic waste water should be treated properly, and all harmful substances should be removed from it, so it can be reused for flushing toilets and gardening.
- Use bio-pesticides (natural pest control) instead of chemical pest control.
- Use compost made from cow dung, garden waste and kitchen waste as a fertiliser.
- Water released from industries should be treated before being discharged or recycled for industrial purpose.
Points to Remember:
- Next to air, water is the most important resource for our survival.
- Water contains hydrogen and oxygen as its constituent elements. Its molecular formula is H2O.
- Water is broken down into its constituent elements by electrolysis. During electrolysis hydrogen and oxygen are obtained in the ratio 2:1.
- Water has a maximum density of 1 g/cc at 4°C. At temperatures below and above 4°C, water has a density of less than 1 g/cc. This unique property of water helps in the survival of aquatic life in winters and summers.
- Sea water contains many minerals and salts dissolved in it and so it is said to be saline.
- Water freezes at 0°C and boils at 100°C.
- Water is a universal solvent as it can dissolve many substances.
- Water that is used for drinking is called potable water.
- Water has dissolved gases which are used by aquatic life for respiration and photosynthesis.
- Hardness of water is due to the presence of dissolved salts of calcium and magnesium.
- Water pollution is the result of dumping untreated domestic solid waste and sewage, agricultural waste, industrial effluents into lakes, rivers, etc.
Glossary:
Electrolysis – Breaking down of substances by the passage of electric current.
Potable water – Water used for drinking.
Saline water – Water containing sodium chloride (common salt)
Sterilization – Addition of chemicals to kill the microorganisms present in water.
Eutrophication – Over growth of algae in water bodies due to excessive fertilizers
Specific heat capacity – Amount of heat that is needed to raise the temperature of a unit mass of a substance by 1°C
Latent heat of fusion – Amount of heat energy required by ice to change into water.
Aeration – The process in which air under pressure is blown into filtered water.
Water pollution – Addition of unwanted waste materials to the water
Domestic sewage – Wastewater that is disposed of from households
Water conservation – Saving water for the use in future
Do You Know?
Henry Cavendish was a British philosopher, scientist, chemist, and physicist. Cavendish is noted for his discovery of hydrogen. He called it inflammable air. He mixed metals with strong acids and created hydrogen. He created carbon dioxide also by combining metals with strong bases.
Pure water has the following physical properties:
- Pure water boils at 100° C at one atmospheric pressure.
- Pure water freezes at exactly 0°C at one atmospheric pressure.
- Pure water has a density of 1 gm/cm3
When the skaters move on ice, they exert pressure on it. This pressure lowers the freezing point. As a result, the ice melts underneath the skate and allows the skaters to glide across the ice with little effort. When the skaters move forward, pressure is decreased and the water re-freezes to ice again.
The freshness of fish and meat can be maintained by placing them in contact with ice. With its larger latent heat, ice is able to absorb a large quantity of heat from the fish as it melts. Thus, food can be kept at a low temperature for an extended period of time.
Copper does not react with water at any temperature. That is why it is used for making pipes and boilers.
Tap water, river water and well water contain dissolved solids but rainwater and distilled water do not contain dissolved solids. Hence concentric rings are not formed in the rain water and distilled water after evaporation.
The salinity of water is more in the Dead Sea. It is actually a salt lake as it has a single source of water and is not connected to the ocean. It is landlocked and this causes the water to evaporate. This has led to a steady increase in its degree of salinity. Now the salinity is so high such that the marine life cannot survive in it. This is why it is called the Dead sea.
Every year 4.6 million children die due to diarrhea. Access to clean water improves hygiene and health.
RO purifiers are the purifiers that can remove the dissolved impurities and germs. They also improve the taste of water. RO stands for the name of the technology, ‘reverse osmosis’, used in these purifiers. Some RO purifiers also have a UV (ultraviolet) unit that destroys the germs present in water.
Distilled water and boiled water have no taste. The pleasant taste of drinking water is due to the presence of dissolved substances which include air, carbon dioxide and minerals.
About 90% of the available surface water has already been tapped mainly for agriculture and irrigation.
The largest source of water pollution in India is untreated sewage. On an average, a person uses 135 litres of water per day for washing clothes, cooking, bathing, etc.
Plastic sheets are used in agriculture to grow vegetables. At the end of the season, these plastic sheets are ploughed back into the soil. The plastic sheets break into tiny pieces and get eaten by earth worms, which is harmful to their health and that of soil.
Micro-plastics can be found in almost every freshwater source. They have been found from the freezing waters of the Arctic and Antarctic to the bottom of the deep-sea floor up to 5,000 meters deep. Micro-plastics have been found in bottled water and tap water around the world.