Thermal Physics Book Back Questions 10th Science Lesson 3
10th Science Lesson 3
3] Thermal Physics
Book Back Questions with Answer and Do You Know Box Content
Choose the best answers:
1. The value of universal gas constant
(a) 3.81 Jmol-1 K-1
(b) 8.03 Jmol-1 K-1
(c) 1.38 Jmol-1 K-1
(d) 8.31Jmol-1 K-1
2. If substance is heated or cooled, the change in mass of that substance is
(a) Positive
(b) Negative
(c) Zero
(d) None of the above
3. If a substance is heated or cooled, the linear expansion occurs along the axis of
(A) X or –X
(b) Y or –Y
(c) Both (a) and (b)
(d) (a) or (b)
4. Temperature is the average ___________ of the molecules of a substance
(a) Difference in K.E and P.E
(b) Sum of P.E and K.E
(c) Difference in T.E and P.E
(d) Difference in K.E and T.E
5. 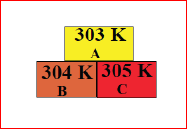 In the given diagram, the possible direction of heat energy transformation is
In the given diagram, the possible direction of heat energy transformation is
(a) A ???? B, A ???? C, B ???? C
(b) A ???? B, A ???? C, B ???? C
(c) A ???? B, A ???? C, B ???? C
(d) A ???? B, A ???? C, B ???? C
Fill in the blanks:
1. The value of Avogadro number _____________.
a. 6.032 x 1023
b. 6.023 x 1023
c. 6.023 x 1025
d. 6.032 x 1025
2. The temperature and heat are _________________ quantities.
a. vector
b. scalar
c. molecular
d. atomic
3. One calorie is the amount of heat energy required to raise the temperature of ________________ of water through ________________.
a. 1mg, 1oC
b. 1kg, 1oC
c. 1g, 1oC
d. 1kg, 10oC
4. According to Boyle’s law, the shape of the graph between pressure and reciprocal of volume is ______________.
a. curve
b. square
c. Parallel
d. straight line
State true or false. If false, explain why?
1. For a given heat in liquid, the apparent expansion is more than that of real expansion.
2. Thermal energy always flows from a system at higher temperature to a system at lower temperature.
3. According to Charles’s law, at constant pressure, the temperature is inversely proportional to volume.
Match the items in column – I to the items in column – II:
Column – I Column – II
1. Linear expansion – change in volume
2. Superficial expansion – hot body to cold body
3. Cubical expansion – 1.381 X 10-23 JK-1
4. Heat transformation – change in length
5. Boltzmann constant – change in area
Consider the statements given below and choose the correct option:
(a) If both assertion and reason are true and reason is the correct explanation of assertion
(b) If both assertion and reason are true but reason is not the correct explanation of assertion
(c) If assertion is true but reason is false
(d) If assertion is false but reason is true
1. Assertion: There is no effect on other end when one end of the rod is only heated.
Reason: Heat always flows from a region of lower temperature to higher temperature of the rod.
2. Assertion: Gas is highly compressible than solid and liquid.
Reason: Inter-atomic or intermolecular distance in the gas is comparably high.
Answers:
Choose the best answer:
1.8.31 J mol-1 K-1 2. Zero 3. X or –X 4. Difference of T.E and P.E 5. A????B, A????C, B????C.
Fill in the Blanks:
1.6.023 X 1023 2. Scalar 3. 1gm, 1oC 4. Straight line
True or False:
1.False
2.True
3.False (At constant pressure, the volume is directly proportional to the temperature)
Match the following:
1. Change in length 2. Change in area 3. Change in volume 4. Hot body to cold body 5. 1.381 X 10-23 JK-1
Assertion and Reason:
1. Assertion is true but reason is false
2. Both assertion and reason are true and reason is the correct explanation of assertion.