Matter Online Test 8th Science Lesson 4 Questions in English
Matter Online Test 8th Science Lesson 4 Questions in English
Matter Online Test 8th Science Lesson 4 Questions in English
Quiz-summary
0 of 49 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
Information
Matter Online Test 8th Science Lesson 4 Questions in English
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 49 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- Answered
- Review
-
Question 1 of 49
1. Question
1. Which of the following statement is incorrect?
1) The various objects which exist around us are made of some kind of matter.
2) In the universe all manifestations, phenomena and evolution of life are caused by matter and energy
Correct
Explanation
In the universe all manifestations, phenomena and evolution of life are caused by matter and energy. The various objects which exist around us are made of some kind of matter.
Incorrect
Explanation
In the universe all manifestations, phenomena and evolution of life are caused by matter and energy. The various objects which exist around us are made of some kind of matter.
-
Question 2 of 49
2. Question
2. Match the following with their respective:
I. Glass tumbler 1. Felt
II. Agarbatti 2. Seen
III. Wind blowing 3. Smell
Correct
Explanation
We perceive some of these objects through our senses like sight, touch, hearing, taste and smelling. A glass tumbler can be seen, agarbatti burning can be recognized by its smell whereas wind blowing can be felt.
Incorrect
Explanation
We perceive some of these objects through our senses like sight, touch, hearing, taste and smelling. A glass tumbler can be seen, agarbatti burning can be recognized by its smell whereas wind blowing can be felt.
-
Question 3 of 49
3. Question
3. Assertion(A): Matter can be defined as anything, which occupies space or volume and mass and be perceived by our senses
Reason(R): All kinds of matter possess mass and occupy space, of course some are heavy and others are light
Correct
Explanation
All kinds of matter possess mass and occupy space, of course some are heavy and others are light. Thus, matter can be defined as anything, which occupies space or volume and mass and can be perceived by our senses.
Incorrect
Explanation
All kinds of matter possess mass and occupy space, of course some are heavy and others are light. Thus, matter can be defined as anything, which occupies space or volume and mass and can be perceived by our senses.
-
Question 4 of 49
4. Question
4. Match the following:
I. Solids 1. Oxygen
II. Liquids 2. Iron
III. Gases 3. Fruit juice
Correct
Explanation
As we know already matter exists in:
Solids: Substances like wood, stone, sand, iron etc.
Liquids: Substances like water, milk, fruit juice, etc
Gases: Substances like oxygen, nitrogen, carbon dioxide, steam, etc.,
Incorrect
Explanation
As we know already matter exists in:
Solids: Substances like wood, stone, sand, iron etc.
Liquids: Substances like water, milk, fruit juice, etc
Gases: Substances like oxygen, nitrogen, carbon dioxide, steam, etc.,
-
Question 5 of 49
5. Question
5. By which of the following a matter is composed of?
1) Atom
2) Molecule
3) Ions
Correct
Explanation
Matter in any physical state is composed of smaller particles such as atom, molecules or ions. Molecules are also made up of atoms of same or different kinds. Hence, atoms are the building blocks of matter.
Incorrect
Explanation
Matter in any physical state is composed of smaller particles such as atom, molecules or ions. Molecules are also made up of atoms of same or different kinds. Hence, atoms are the building blocks of matter.
-
Question 6 of 49
6. Question
6. Which of the following statement about atom is correct?
1) An atom is the smallest particle of an element, which exhibits all the properties of that element.
2) It may or may not exist independently but takes part in every chemical reaction
Correct
Explanation
An atom is the smallest particle of an element, which exhibits all the properties of that element. It may or may not exist independently but takes part in every chemical reaction.
Incorrect
Explanation
An atom is the smallest particle of an element, which exhibits all the properties of that element. It may or may not exist independently but takes part in every chemical reaction.
-
Question 7 of 49
7. Question
7. _____is the smallest particle of a pure substance
Correct
Explanation
Atoms of the same element or different elements combine to form a molecule. A molecule is the smallest particle of a pure substance (element or compound), which can exist independently and retain the physical and chemical properties of the substance.
Incorrect
Explanation
Atoms of the same element or different elements combine to form a molecule. A molecule is the smallest particle of a pure substance (element or compound), which can exist independently and retain the physical and chemical properties of the substance.
-
Question 8 of 49
8. Question
8. Who used symbols to represent the four basic elements around us?
Correct
Explanation
The symbols in form of the geometrical shapes were those used by the ancient Greeks to represent the four basic elements around us such as earth, air, fire and water.
Incorrect
Explanation
The symbols in form of the geometrical shapes were those used by the ancient Greeks to represent the four basic elements around us such as earth, air, fire and water.
-
Question 9 of 49
9. Question
9. Which of the following statement is correct?
1) In the days of alchemists, the different materials that they used were represented by the above-mentioned symbols while they try to change less valuable metal into gol
2) The process was called alchemy and the men who did this work were known as alchemists
Correct
Explanation
In the days of alchemists, the different materials that they used were represented by the above- mentioned symbols while they try to change less valuable metal into gold. The process was called alchemy and the men who did this work were known as alchemists.
Incorrect
Explanation
In the days of alchemists, the different materials that they used were represented by the above- mentioned symbols while they try to change less valuable metal into gold. The process was called alchemy and the men who did this work were known as alchemists.
-
Question 10 of 49
10. Question
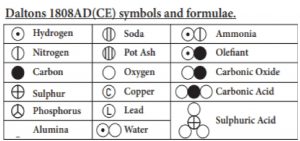
10. Which English scientist tried to name the various elements based on these pictorial symbols?
Correct
Explanation
In 1808, John Dalton, English scientist tried to name the various elements based on these pictorial symbols. These symbols are difficult to draw and hence they are not used. It is only of historical importance.
Incorrect
Explanation
In 1808, John Dalton, English scientist tried to name the various elements based on these pictorial symbols. These symbols are difficult to draw and hence they are not used. It is only of historical importance.
-
Question 11 of 49
11. Question
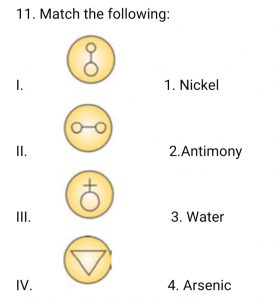 Correct
Correct
Explanation
 Incorrect
Incorrect
Explanation

-
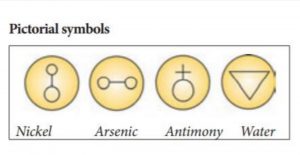
Question 12 of 49
12. Question
 Correct
Correct
Explanation
 Incorrect
Incorrect
Explanation

-
Question 13 of 49
13. Question
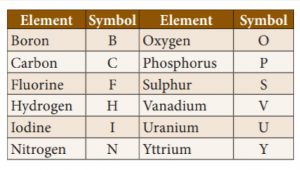
13. Who devised a system using letters of alphabet rather than signs?
Correct
Explanation
In 1813, Jon Jakob Berzelius devised a system using letters of alphabet rather than signs.,. The modified version of Berzelius system follows under the heading ‘System for Determining Symbols of the Elements’.
Incorrect
Explanation
In 1813, Jon Jakob Berzelius devised a system using letters of alphabet rather than signs.,. The modified version of Berzelius system follows under the heading ‘System for Determining Symbols of the Elements’.
-
Question 14 of 49
14. Question
14. What does the symbol B represent?
Correct
Explanation
 Incorrect
Incorrect
Explanation

-
Question 15 of 49
15. Question
15. What does the term Ba represent?
Correct
Explanation
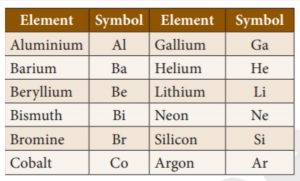 Incorrect
Incorrect
Explanation

-
Question 16 of 49
16. Question
16. What does the symbol As represent?
Correct
Explanation
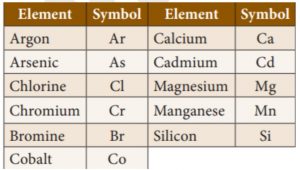 Incorrect
Incorrect
Explanation

-
Question 17 of 49
17. Question
17. Which of the following metal’s symbol does not come from its Latin name?
Correct
Explanation
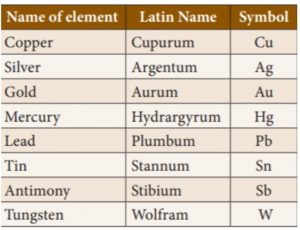 Incorrect
Incorrect
Explanation

-
Question 18 of 49
18. Question
18. Which of the following metal is named after Alfred Nobel?
Correct
Explanation
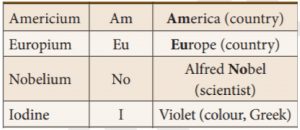 Incorrect
Incorrect
Explanation

-
Question 19 of 49
19. Question
19. Which of the following planet name is not used to name an element?
Correct
Explanation
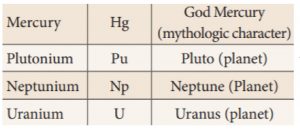 Incorrect
Incorrect
Explanation

-
Question 20 of 49
20. Question
20. What does the symbol O represent?
Correct
Explanation
Symbol of an element signifies:
Name of the element
One atom of the element
For example, the symbol O stands for the element of Oxygen, One atom of oxygen
Incorrect
Explanation
Symbol of an element signifies:
Name of the element
One atom of the element
For example, the symbol O stands for the element of Oxygen, One atom of oxygen
-
Question 21 of 49
21. Question
21. Which of the following statement is correct?
1) The progress of man towards civilization is linked with the discovery of several metals and non-metals
2) Even today, the index of prosperity of a country depends upon the amount of metals and non-metals it produces and uses
Correct
Explanation
The progress of man towards civilization is linked with the discovery of several metals and non- metals. Even today, the index of prosperity of a country depends upon the amount of metal and non-metals it produces and uses.
Incorrect
Explanation
The progress of man towards civilization is linked with the discovery of several metals and non- metals. Even today, the index of prosperity of a country depends upon the amount of metal and non-metals it produces and uses.
-
Question 22 of 49
22. Question
22. The wealth of a country is measured by the amount of____ in its reserve.
Correct
Explanation
The wealth of a country is measured by the amount of gold in its reserve. These days, metals and non-metals are used for making tools, machines, cars, utensils, etc. Some of the common metals used are iron, copper, silver, gold, lead, zinc, aluminium, magnesium, nickel, chromium and mercury etc. Similarly, the common non-metals used are nitrogen, oxygen, hydrogen, carbon, sulphur, phosphorus and chlorine etc.
Incorrect
Explanation
The wealth of a country is measured by the amount of gold in its reserve. These days, metals and non-metals are used for making tools, machines, cars, utensils, etc. Some of the common metals used are iron, copper, silver, gold, lead, zinc, aluminium, magnesium, nickel, chromium and mercury etc. Similarly, the common non-metals used are nitrogen, oxygen, hydrogen, carbon, sulphur, phosphorus and chlorine etc.
-
Question 23 of 49
23. Question
23. Which of the following statement is incorrect?
1) An element can be identified as metal or non-metal by comparing its properties with the general properties of metals and non- metals.
2) In doing so, we find that some elements neither fit with the metals or with non-metals, they are called as metalloid
Correct
Explanation
An element can be identified as metal or non-metal by comparing its properties with the general properties of metals and non- metals. In doing so, we find that some elements neither fit with the metals or with non-metals. Such elements are called semi-metals or metalloids. Elements are classified into metals, non-metals, and metalloids based on their properties.
Incorrect
Explanation
An element can be identified as metal or non-metal by comparing its properties with the general properties of metals and non- metals. In doing so, we find that some elements neither fit with the metals or with non-metals. Such elements are called semi-metals or metalloids. Elements are classified into metals, non-metals, and metalloids based on their properties.
-
Question 24 of 49
24. Question
24. Which of the following metals become liquid at or just above room temperature?
Correct
Explanation
Metals are solid under normal conditions of temperature and pressure. Mercury is liquid at room temperature. Elements Caesium (Cs), rubidium (Rb), Francium (Fr) and Gallium (Ga) become liquid at or just above room temperature
Incorrect
Explanation
Metals are solid under normal conditions of temperature and pressure. Mercury is liquid at room temperature. Elements Caesium (Cs), rubidium (Rb), Francium (Fr) and Gallium (Ga) become liquid at or just above room temperature
-
Question 25 of 49
25. Question
25. _____is so hard that it can scratch glass
Correct
Most metals are hard. The exception here is sodium and potassium, which is soft enough to be cut by a knife. Osmium is so hard that it can scratch glass.
Incorrect
Most metals are hard. The exception here is sodium and potassium, which is soft enough to be cut by a knife. Osmium is so hard that it can scratch glass.
-
Question 26 of 49
26. Question
26. Which of the following metal does not Lustre?
Correct
Explanation
All metals are shiny. Th e typical shine of metals is called metallic lustre. All metals have a typical metallic lustre. An exception is calcium.
Incorrect
Explanation
All metals are shiny. Th e typical shine of metals is called metallic lustre. All metals have a typical metallic lustre. An exception is calcium.
-
Question 27 of 49
27. Question
27. Which of the following metal have low density?
1) Sodium
2) Zinc
3) Potassium
Correct
Explanation
Metals generally have high density. Sodium and potassium have exceptionally low density. All metals have a typical metallic lustre.
Incorrect
Explanation
Metals generally have high density. Sodium and potassium have exceptionally low density. All metals have a typical metallic lustre.
-
Question 28 of 49
28. Question
28. Which metal does not have high melting point and boiling point?
1) Sodium
2) Potassium
3) Mercury
4) Gallium
Correct
Explanation
Melting point and boiling point: Metals in general have high melting point and boiling point. Sodium, potassium, mercury and gallium are exceptions.
Incorrect
Explanation
Melting point and boiling point: Metals in general have high melting point and boiling point. Sodium, potassium, mercury and gallium are exceptions.
-
Question 29 of 49
29. Question
29. Which of the following statement is correct?
1) Metals have the capacity to withstand strain without breaking
2) It is the property that owes the use of iron for the construction of railway tracks
3) Zinc, arsenic and antimony are exceptions.
Correct
Explanation
Metals have the capacity to withstand strain without breaking. This property is called tensile strength. It is the property that owes the use of iron for the construction of railway tracks. Zinc, arsenic and antimony are exceptions
Incorrect
Explanation
Metals have the capacity to withstand strain without breaking. This property is called tensile strength. It is the property that owes the use of iron for the construction of railway tracks. Zinc, arsenic and antimony are exceptions
-
Question 30 of 49
30. Question
30. Which metal have the property to transform into silvery foils?
Correct
Explanation
Metals can be hammered into very thin sheets. Th is tendency of metals is called malleability. Aluminium makes use of this property to transform into silvery foils.
Incorrect
Explanation
Metals can be hammered into very thin sheets. Th is tendency of metals is called malleability. Aluminium makes use of this property to transform into silvery foils.
-
Question 31 of 49
31. Question
31. Property of making metals into thin wire is called_________
Correct
Explanation
Ductility: Metals can be drawn into thin wires. This property of metals is called ductility. Example: copper wires.
Incorrect
Explanation
Ductility: Metals can be drawn into thin wires. This property of metals is called ductility. Example: copper wires.
-
Question 32 of 49
32. Question
32. Which of the following metal is/are poor conductor of electricity?
Correct
Explanation
Metals are good conductors of heat and electricity. Silver and copper are very good conductors of electricity. However, bismuth and tungsten are poor conductors.
Incorrect
Explanation
Metals are good conductors of heat and electricity. Silver and copper are very good conductors of electricity. However, bismuth and tungsten are poor conductors.
-
Question 33 of 49
33. Question
33. _____ property is being made used in making temple bells
Correct
Explanation
On being hit, metals produce a typical sound. Hence, they are said to be sonorous. This property is being made used in making temple bells.
Incorrect
Explanation
On being hit, metals produce a typical sound. Hence, they are said to be sonorous. This property is being made used in making temple bells.
-
Question 34 of 49
34. Question
34. Which of the following are non-metals?
1) Sulphur
2) Carbon
3) Oxygen
Correct
Explanation
Elements that generally do not shine, neither too hard nor too soft, are non-metals. All gases are non-metals. Some non-metals are Sulphur, Carbon, Oxygen etc.
Incorrect
Explanation
Elements that generally do not shine, neither too hard nor too soft, are non-metals. All gases are non-metals. Some non-metals are Sulphur, Carbon, Oxygen etc.
-
Question 35 of 49
35. Question
35. Which of the following non-metal occurs in solid state?
Correct
Explanation
Non-metals occur as solids, liquids or gases at normal temperature, for example sulphur, phosphorus occurs in solid state while bromine occurs in liquid state. Gases like oxygen, nitrogen, etc., occur in the gaseous state.
Incorrect
Explanation
Non-metals occur as solids, liquids or gases at normal temperature, for example sulphur, phosphorus occurs in solid state while bromine occurs in liquid state. Gases like oxygen, nitrogen, etc., occur in the gaseous state.
-
Question 36 of 49
36. Question
36. Which of the following statement is correct?
1) Non-metals are generally not hard except diamond
2) Non-metals have a dull appearance; Graphite and iodine are exceptions as they are shiny and lustrous
Correct
Explanation
Non-metals are generally not hard except diamond (a form of carbon). Non-metals have a dull- appearance; Graphite and iodine are exceptions as they are shiny.
Incorrect
Explanation
Non-metals are generally not hard except diamond (a form of carbon). Non-metals have a dull- appearance; Graphite and iodine are exceptions as they are shiny.
-
Question 37 of 49
37. Question
37. Which of the following non-metals have high melting and boiling point?
1) Carbon
2) Silicon
3) Boron
Correct
Explanation
Nonmetals have low melting point and boiling point. However, carbon, silicon and boron are exceptions. Non-metals do not have tensile strength. However, carbon fibre (a form of carbon) is as tensile as steel.
Incorrect
Explanation
Nonmetals have low melting point and boiling point. However, carbon, silicon and boron are exceptions. Non-metals do not have tensile strength. However, carbon fibre (a form of carbon) is as tensile as steel.
-
Question 38 of 49
38. Question
38. Which of the following metal properties are usually high?
1) Melting point
2) Boiling point
3) Density
Correct
Explanation
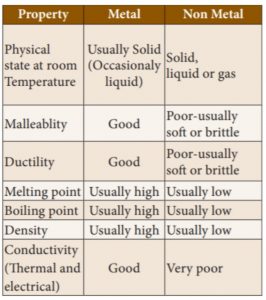 Incorrect
Incorrect
Explanation

-
Question 39 of 49
39. Question
39. ________ is used in electrical wires, cables and in aerospace industries
Correct
Explanation
Uses of Metals:
Iron is used for making bridges, engine parts, iron-sheet and bars.
Copper is used for making electrical wires, coins and statue.
Silver and gold are used for making jewels, in decorative purposes and photography
Mercury is used in thermometers and barometers because of its high density and uniform expansion at different temperature.
Aluminium is used in electrical wires, cables and in aerospace industries.
Incorrect
Explanation
Uses of Metals:
Iron is used for making bridges, engine parts, iron-sheet and bars.
Copper is used for making electrical wires, coins and statue.
Silver and gold are used for making jewels, in decorative purposes and photography
Mercury is used in thermometers and barometers because of its high density and uniform expansion at different temperature.
Aluminium is used in electrical wires, cables and in aerospace industries.
-
Question 40 of 49
40. Question
40. _________ is used in making pencil lead.
Correct
Explanation
Diamond (a form of carbon) is used for making jewels, cutting and grinding equipment. Graphite is used in making pencil lead.
Incorrect
Explanation
Diamond (a form of carbon) is used for making jewels, cutting and grinding equipment. Graphite is used in making pencil lead.
-
Question 41 of 49
41. Question
41. _______ is used in the manufacturing of gun powder
Correct
Explanation
Sulphur is used in the manufacturing of gun powder and vulcanization of rubber. Phosphorus is used in matches, rat poison etc.
Incorrect
Explanation
Sulphur is used in the manufacturing of gun powder and vulcanization of rubber. Phosphorus is used in matches, rat poison etc.
-
Question 42 of 49
42. Question
42. _____ is used as a bleaching agent and in sterilizing water.
Correct
Explanation
Nitrogen is used for manufacturing ammonia. Chlorine is used as a bleaching agent and in sterilizing water. Hydrogen is used as a rocket fuel and hydrogen flame is used for cutting and welding purposes, as well as a reducing agent.
Incorrect
Explanation
Nitrogen is used for manufacturing ammonia. Chlorine is used as a bleaching agent and in sterilizing water. Hydrogen is used as a rocket fuel and hydrogen flame is used for cutting and welding purposes, as well as a reducing agent.
-
Question 43 of 49
43. Question
43. Which of the following is/are Metalloids?
Correct
Explanation
The elements which exhibit the properties of metals as well as non-metals are called metalloids. Examples: boron, silicon, arsenic, germanium, antimony, tellurium and polonium.
Incorrect
Explanation
The elements which exhibit the properties of metals as well as non-metals are called metalloids. Examples: boron, silicon, arsenic, germanium, antimony, tellurium and polonium.
-
Question 44 of 49
44. Question
44. Which of the following are the Physical properties of metalloids?
1) They can form alloys with other metals
2) The physical properties of metalloids tend to be metallic, but their chemical properties tend to be non-metallic.
3) Silicon for example appears lustrous, but is not malleable nor ductile (it is brittle – a characteristic of some non-metals)
Correct
Explanation
Physical properties of metalloids:
Metalloids are all solid at room temperature
They can form alloys with other metals
Some metalloids, such as silicon and germanium, can act as electrical conductors under the specific conditions, thus they are called semiconductors.
Silicon for example appears lustrous, but is not malleable nor ductile (it is brittle – a characteristic of some non-metals). It is a much poorer conductor of heat and electricity than the metals
The physical properties of metalloids tend to be metallic, but their chemical properties tend to be non-metallic
Incorrect
Explanation
Physical properties of metalloids:
Metalloids are all solid at room temperature
They can form alloys with other metals
Some metalloids, such as silicon and germanium, can act as electrical conductors under the specific conditions, thus they are called semiconductors.
Silicon for example appears lustrous, but is not malleable nor ductile (it is brittle – a characteristic of some non-metals). It is a much poorer conductor of heat and electricity than the metals
The physical properties of metalloids tend to be metallic, but their chemical properties tend to be non-metallic
-
Question 45 of 49
45. Question
45. What is the ratio of mass of water oxygen and hydrogen atoms?
Correct
Explanation
Water, carbon di oxide, sodium chloride etc. are few examples of compounds. A molecule of water is composed of an oxygen atom and two hydrogen atoms in the ratio 1:2 by volume or 8:1 by mass.
Incorrect
Explanation
Water, carbon di oxide, sodium chloride etc. are few examples of compounds. A molecule of water is composed of an oxygen atom and two hydrogen atoms in the ratio 1:2 by volume or 8:1 by mass.
-
Question 46 of 49
46. Question
46. Which of the are the Constitutent Elements of Zinc carbonate?
1) Zinc
2) Carbon
3) Sulphur
4) Oxygen
Correct
Explanation
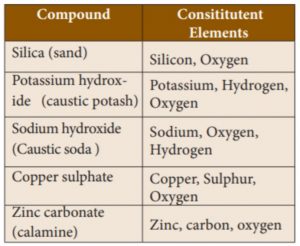 Incorrect
Incorrect
Explanation

-
Question 47 of 49
47. Question
47. What is the Consititutent Elements of Vinegar?
1) Hydrogen
2) Carbon
3) Oxygen
4) Sulphur
Correct
Explanation
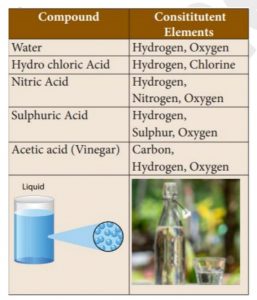 Incorrect
Incorrect
Explanation

-
Question 48 of 49
48. Question
48. Match the following:
I. Baking soda 1. Calcium hydroxide
II. Quick lime 2. Sodium bicarbonate
III. Slaked lime 3. Calcium oxide
IV. Bleaching powder 4. Calcium hydroxide
Correct
Explanation
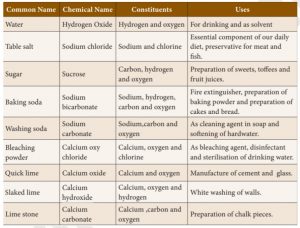 Incorrect
Incorrect
Explanation

-
Question 49 of 49
49. Question
49. Match the following:
I. Copper sulphate 1. Blue Vitriol
II. Calcium sulphate 2. Gypsum
III. Potassium chloride 3. Muriate of potash
IV. Sulphuric acid 4. Oil of Vitriol
Correct
Explanation
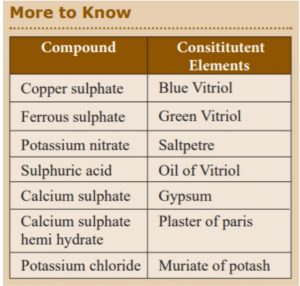 Incorrect
Incorrect
Explanation

Leaderboard: Matter Online Test 8th Science Lesson 4 Questions in English
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||