Matter Around Us Notes 8th Science Lesson 9 Notes in English
Matter Around Us Notes 8th Science Lesson 9 Notes in English
In the universe all manifestations, phenomena and evolution of life are caused by matter and energy. All the objects which exist around us are made of some kind of matter. We perceive these objects through our senses like sight, touch, hearing, taste and smelling. A glass tumbler can be seen, agarbatti burning can be recognized by its smell whereas wind blowing can be felt. All kinds of matter possess mass and occupy space. Of course some are heavy and others are light. Thus, matter can be defined as anything which has definite mass
and occupies space.
As we know already, matter exists as solids (wood, stone, sand, iron etc.), liquids (water, milk, fruit juice, etc.) and gases (oxygen, nitrogen, carbon dioxide, steam, etc.). Matter in any physical state is composed of smaller particles such as atom, molecules or ions. An atom is the smallest particle of an element which exhibits all the properties of that element. Atoms of the same element or different elements combine to form a molecule. Atoms or group of atoms having a charge (positive or negative ) are called ions.
Hence, atoms are the building blocks of matter. In this lesson, we will study about symbols of elements, metals and non metals, compounds of solids, liquids and gases, and the uses of compounds in daily life.
Elements:
Elements are everywhere. They are the building blocks of everything on Earth: pencil, desk, mountains, car, book, etc. Do you know that when you are breathing you are actually inhaling air? The air you breathe is made up of many elements like oxygen, nitrogen and argon.
An element is a pure substance that cannot be broken down by chemical methods into simpler components. For example, the element gold cannot be broken down into anything other than gold. If you keep hitting gold with a hammer, the pieces would get smaller, but each piece will always be gold.
Elements consist of only one type of atoms. An atom is the smallest particle of an element that still has the same properties of that element.
All atoms of a specific element have exactly the same chemical makeup, size, and mass. Each atom has an atomic number, which represents the number of protons that are in the nucleus of a single atom of that element. There are a total of 118 elements. Many elements occur naturally on Earth; however, some are created in laboratory by scientists.
Symbols of Elements:
A symbol is an image, object, etc., that stands for some meaning. For instance, a dove is a symbol of peace. Similarly, we denote mathematical operations by symbols. For example ‘+’ denotes addition; ‘–’ denotes subtraction etc. In the same way in chemistry each element is denoted by a symbol. Writing out the name of an element every time would become too troublesome. So, the name of an element is represented by shortened form called as symbol. Let us learn the brief history of symbols of elements.
- Greek Symbols:
The symbols in the form of geometrical shapes were used by the ancient Greeks to represent the four basic elements around us such as earth, air, fire and water.
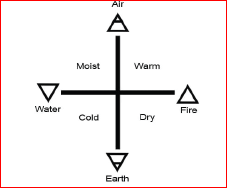
Greek symbols
- Alchemist Symbols:
In the days of alchemists, different materials that people used were represented by different symbols while they tried to change less valuable metal into gold. That process was called alchemy and the men who did this work were known as alchemists.
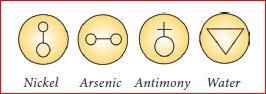
Symbols used by Alchemist
- Dalton Symbols:
In 1808, John Dalton, English scientist tried to name various elements based on pictorial symbols. Those symbols are difficult to draw and hence they are not used. It is only of historical importance.
Dalton Symbols
| Hydrogen | Copper |
| Nitrogen | Lead |
| Carbon | Water |
| Sulphur | Ammonia |
| Phosphorus | Olefiant |
| Alumina | Carbonic oxide |
| Soda | Carbonic acid |
| Pot ash | Sulphuric acid |
| Oxygen |
- Berzelius Symbols:
In 1813, Jon Jakob Berzelius devised a system using letters of alphabet rather than signs. The modified version of Berzelius system follows under the heading ‘System for Determining Symbols of the Elements’.
- Present system for determining symbols of the elements:
- The symbols of the most common elements, mainly non-metals, use the first letter of their English name.
Elements having first letter as symbol
| Element | Symbol | Element | Symbol |
| Boron | B | Oxygen | O |
| Carbon | C | Phosphorus | P |
| Fluorine | F | Sulphur | S |
| Hydrogen | H | Vanadium | V |
| Iodine | I | Uranium | U |
| Nitrogen | N | Yttrium | Y |
- If two elements have same first letter, then the first and second letters of the name are used as symbols. The first letter is in uppercase and the second letter is in lowercase.
Elements with same first letter
| Element | Symbol | Element | Symbol |
| Aluminium | Al | Hydrogen | H |
| Barium | Ba | Helium | He |
| Beryllium | Be | Nickel | Ni |
| Bismuth | Bi | Neon | Ne |
| Bromine | Br | Silicon | Si |
| Cobalt | Co | Sulphur | S |
- If the first two letters of the names of the elements are same, then the symbol consists of first letter and second or third letter of English name that they do not have in common.
Elements with same two letters
| Element | Symbol | Element | Symbol |
| Argon | Ar | Calcium | Ca |
| Arsenic | As | Cadmium | Cd |
| Chlorine | Cl | Magnesium | Mg |
| Chromium | Cr | Manganese | Mn |
- Some symbols are used on the basis of their Greek name or Latin name of the elements. There are eleven such elements.
Greek or Latin name of elements
| Element | Latin Name | Symbol |
| Sodium | Natrium | Na |
| Potassium | Kalium | K |
| Iron | Ferrum | Fe |
| Copper | Cuprum | Cu |
| Silver | Argentum | Ag |
| Gold | Aurum | Au |
| Mercury | Hydrargyrum | Hg |
| Lead | Plumbum | Pb |
| Tin | Stannum | Sn |
| Antimony | Stibium | Sb |
| Tungsten | Wolfram | W |
- Some elements are named using the name of the country / scientist / colour / mythological character / planet.
Elements named using name of the country, scientist etc.
| Name | Symbol | Name derived from |
| Americium | Am | America (Country) |
| Europium | Eu | Europe (Country) |
| Nobelium | No | Alfred Nobel (Scientist) |
| Iodine | I | Violet (Colour, Greek) |
| Mercury | Hg | God Mercury (Mythologic character) |
| Plutonium | Pu | Pluto (Planet) |
| Neptunium | Np | Neptune (Planet) |
| Uranium | U | Uranus (planet) |
Writing the Symbols:
While writing the symbol for an element, we should adhere to the following rules.
- If the element has a single English letter as a symbol, it should be written in capital letter.
- For elements having two letter symbols, the first letter should be in capital followed by small letter
Metals and Non-metals:
The progress of man towards civilization is linked with the discovery of several metals and non-metals. Even today, the index of prosperity of a country depends upon the amount of metals and non-metals it produces. The wealth of a country is measured by the amount of gold in its reserve.
An element can be identified as metal or non-metal by comparing its properties with the general properties of metals and non-metals. In doing so, we find that some elements neither fit with the metals nor with non-metals. Such elements are called semimetals or metalloids. Elements are classified into metals, non-metals, and metalloids based on their properties.

Metals:
Iron, copper, gold, silver, etc. that we use in our daily life are metals. The properties and uses of metals are given below.
- Physical properties of Metals:
- Metals are solid under normal conditions of temperature and pressure.
- Most metals are hard.
- All metals are shiny. The typical shine of metals is called metallic lustre.
- Metals generally have high density.
- Metals in general have high melting point and boiling point.
- Metals can be hammered into very thin sheets. This tendency of metals is called malleability. Using this property aluminum is transformed into silvery foils.
- Metals can be drawn into thin wires. This property of metals is called ductility. Example: Copper wires.
- Generally metals are good conductors of heat and electricity.
- On being hit, metals produce a typical sound. Hence, they are said to be sonorous.This property is being made used in making temple bells.
Shining metal
- Uses of Metals:
- Iron is used for making bridges, engine parts, iron-sheets and bars.
- Copper is used for making electrical wires, coins and statue.
- Silver and gold are used for making jewels, and for decorative purposes and photography.
- Mercury is used in thermometers and barometers because of its high density and uniform expansion at different temperature.
- Aluminium is used in electrical wires, cables and in aerospace industries.
- Lead is used in automobile batteries, X-ray machines.
Non-metals:
Elements like sulphur, carbon, oxygen etc. are non-metals. Some of the properties and uses of non-metals are given below.
- Properties of Non-metals:
- Non-metals occur as solids, liquids or gases at normal temperature. For example, sulphur and phosphorus occur in solid state while bromine occurs in liquid state. Elements like oxygen, nitrogen etc., occur in gaseous state.
- Non-metals are generally not hard except diamond ( a form of carbon).
- Non-metals have a dull appearance.
- Non-metals are generally soft and have low densities. The exception here is diamond (a form of carbon) which is the hardest naturally occurring substance.
- Non-metals have low melting point and boiling point.
- Non-metals are non-malleable.
- Non-metals are not ductile. Carbon fibre is highly ductile.
- Non-metals are generally bad conductors of electricity. Graphite (a form of carbon) is an exception.
- Non-metals do not produce sound (nonsonorous) when hit.
- Uses of Non-metals:
- Diamond (a form of carbon) is used for making jewels, cutting and grinding equipments. Graphite is used in making pencil lead.
- Sulphur is used in the manufacturing of gun powder and vulcanization of rubber.
- Phosphorus is used to make match boxes, rat poison etc.
- Nitrogen is used for manufacturing ammonia.
- Chlorine is used as a bleaching agent and in sterilizing water.
- Hydrogen is used as a rocket fuel and hydrogen flame is used for cutting and welding purposes. Hydrogen is also used as a reducing agent.
Diamond
Difference between Metals and Non-metals
| Property | Metal | Non Metal |
| Physical state at room temperature | Usually solid (Occasionally liquid) | Solid, liquid or gas |
| Malleablity | Good | Poor (Usually soft or brittle) |
| Ductility | Good | Poor (Usually soft or brittle) |
| Melting point | Usually high | Usually low |
| Boiling point | Usually high | Usually low |
| Density | Usually high | Usually low |
| Conductivity (Thermal and Electrical) | Good | Very poor |
Metalloids:
The elements which exhibit the properties of metals as well as non-metals are called metalloids. Examples: Boron, Silicon, Arsenic, Germanium, Antimony, Tellurium and Polonium.
- Physical properties of Metalloids:
- Metalloids are solids at room temperature.
- They can form alloys with other metals.
- Some metalloids, such as silicon and germanium, can act as electrical conductors under specific conditions. Thus, they are called semiconductors.
- Silicon which is a metalloid appears lustrous, but it is neither malleable nor ductile. It is brittle – a characteristic of some non metals. It is a much poorer conductor of heat and electricity than the metals.
- The physical properties of metalloids tend to be metallic, but their chemical properties tend to be non-metallic.
- Uses of Metalloids:
- Silicon is used in electronic devices.
- Boron is used in fireworks and as a fuel for ignition in rocket.
Compounds:
A compound is a pure substance which is formed due to the chemical combination of two or more elements in a fixed ratio by mass. The properties of a compound are different from those of its constituents. Water, carbon dioxide, sodium chloride etc. are few examples of compounds. A molecule of water is composed of one oxygen atom and two hydrogen atoms in the ratio 1:2 by volume or 8:1 by mass.
Classification of Compounds:
Based on the origin of chemical constituents, compounds are classified as inorganic compounds and organic compounds.
- Inorganic compounds:
Compounds obtained from non living sources such as rock, minerals etc., are called inorganic compounds. Example: Chalk, baking powder etc.,
- Organic compounds:
Compounds obtained from living sources such as plants, animals etc., are called organic compounds. Example: Protein, carbohydrates, etc.,
Both inorganic and organic compounds exist in all three states ie., solids, liquids and gases. Let us learn about some important compounds in solid, liquid and gaseous states.
Some compounds that exist in solid state are given.
Compounds in solid state
| Compounds | Constituent Elements |
| Silica (Sand) | Silicon, Oxygen |
| Potassium hydroxide (Caustic potash) | Potassium, Hydrogen, Oxygen |
| Sodium hydroxide (Caustic soda ) | Sodium, Oxygen, Hydrogen |
| Copper sulphate | Copper, Sulphur, Oxygen |
| Zinc carbonate (Calamine) | Zinc, Carbon, Oxygen |
Compounds which exist in liquid state are given.
Compounds in liquid state
| Compounds | Constituent Elements |
| Water | Hydrogen, Oxygen |
| Hydrochloric acid | Hydrogen, Chlorine |
| Nitric acid | Hydrogen, Nitrogen, Oxygen |
| Sulphuric acid | Hydrogen, Sulphur, Oxygen |
| Acetic acid (Vinegar) | Carbon, Hydrogen, Oxygen |
Some compounds exist in gaseous state also. They are given.
Compounds in gaseous state
| Compounds | Constituent Elements |
| Carbon dioxide, carbon monoxide | Carbon, Oxygen |
| Sulphur dioxide | Sulphur, Oxygen |
| Methane | Carbon, Hydrogen |
| Nitrogen dioxide | Nitrogen, Oxygen |
| Ammonia | Nitrogen, Hydrogen |
Uses of Compounds:
We use a number of compounds in our daily life. Some of them are listed in table.
Uses of Compounds
| Common Name | Chemical Name | Constituents | Uses |
| Water | Dihydrogen monoxide | Hydrogen and Oxygen | For drinking and as solvent. |
| Table salt | Sodium chloride | Sodium and Chlorine | Essential component of our daily diet, preservative for meat and fish. |
| Sugar | Sucrose | Carbon, Hydrogen and Oxygen | Preparation of sweets, toffees and fruit juices. |
| Baking soda | Sodium bicarbonate | Sodium, Hydrogen, Carbon and Oxygen | Fire extinguisher, preparation of baking powder and preparation of cakes and bread. |
| Washing soda | Sodium carbonate | Sodium, Carbon and Oxygen | As cleaning agent in soap and softening of hard water. |
| Bleaching powder | Calcium oxy chloride | Calcium, Oxygen and Chlorine | As bleaching agent, disinfectant and sterilisation of drinking water. |
| Quick lime | Calcium oxide | Calcium and Oxygen | Manufacture of cement and glass. |
| Slaked lime | Calcium hydroxide | Calcium, Oxygen and Hydrogen | White washing of walls. |
| Lime stone | Calcium carbonate | Calcium, Carbon and Oxygen | Preparation of chalk pieces. |
Points to Remember:
- Anything which occupies space and has mass is called matter.
- Material which has a definite shape and definite volume at room temperature with any number of free surfaces is called solid.
- The molecule of a substance that contains two or more atoms of different elements combined together in a definite ratio, is said to be a molecule of a compound.
- Material which has a definite volume, but no definite shape and has one free surface, is called liquid.
- Material which has neither definite shape nor definite volume, is easily compressible and has no free surface is called gas.
- Metals are elements that are hard and shiny in appearance. Some metals used in our daily life are iron, copper, gold, silver, etc. Metals conduct heat and electricity.
- Elements that generally do not shine, that are neither too hard nor too soft are non-metals. All gases are non-metals. Some non-metals are sulphur, carbon, oxygen etc.
- Elements which have the properties of metal and non-metals are called metalloids. Some examples are arsenic, germanium etc.
- On being hit, metals produce a typical sound. They are said to be sonorous. This property is being made used in making temple bells.
- The easiest way to represent the element and to write the chemical formula is using symbols.
Glossary:
Disinfectant – Chemical substance which kills or prevents the disease causing microorganism.
Semiconductor – Substance which acts as bad conductor at low temperature and as good conductor at high temperature.
Reducing – agent Substance which undergoes oxidation reaction.
Carbohydrate – Compound which contains carbon, hydrogen and oxygen.
Bleaching agent – Substance which is used to remove the colour
Preservative – Substance which prevents food being spoiled by microorganism.