Changes Around Us Online Test 7th Science Lesson 9 Questions in English
Changes Around Us Online Test 7th Science Lesson 9 Questions in English
Changes Around Us Online Test 7th Science Lesson 9 Questions in English
Quiz-summary
0 of 54 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
Information
Changes Around Us Online Test 7th Science Lesson 9 Questions in English
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 54 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- Answered
- Review
-
Question 1 of 54
1. Question
1. Which of the following is/are physical change?
1) Ice melts on heating
2) Water starts evaporating
3) Freezing of waterCorrect
Explanation
Changes take place around us all the time. A change refers to an alteration in physical properties
or alteration in the composition of matter. For example, ice melts on heating, that is, it changes
from a solid to liquid. On further heating, water starts evaporating; it changes from a liquid to gas.
Here, there is a change in the physical state of the substanceIncorrect
Explanation
Changes take place around us all the time. A change refers to an alteration in physical properties
or alteration in the composition of matter. For example, ice melts on heating, that is, it changes
from a solid to liquid. On further heating, water starts evaporating; it changes from a liquid to gas.
Here, there is a change in the physical state of the substance -
Question 2 of 54
2. Question
2. Which of the following are altered by physical changes?
1) Colour
2) Texture
3) State of the substanceCorrect
Explanation
Let us look at another change, that is, when objects made of iron are exposed to moist conditions,
a reddish-brown new substance called rust forms on the surface of these objects. In this instance
of rusting, there is change in the composition of the substance. Thus, the physical changes involve
an alteration in the properties such as colour, texture and the state of the substance since there is
formation of a new substance.Incorrect
Explanation
Let us look at another change, that is, when objects made of iron are exposed to moist conditions,
a reddish-brown new substance called rust forms on the surface of these objects. In this instance
of rusting, there is change in the composition of the substance. Thus, the physical changes involve
an alteration in the properties such as colour, texture and the state of the substance since there is
formation of a new substance. -
Question 3 of 54
3. Question
3. Which of the following is chemical change?
1) Butter melting
2) Nail rusting
3) Bread makingCorrect
Explanation
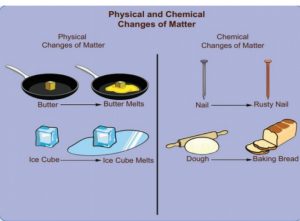 Incorrect
Incorrect
Explanation

-
Question 4 of 54
4. Question
4. Which of the following gas is liberated when paper is burnt?
Correct
Explanation
In case of burning of paper, changes to carbon dioxide and other substances. Now we cannot get
back the paper after burning. As there is a change in the chemical nature, it is called as chemical
change.Incorrect
Explanation
In case of burning of paper, changes to carbon dioxide and other substances. Now we cannot get
back the paper after burning. As there is a change in the chemical nature, it is called as chemical
change. -
Question 5 of 54
5. Question
5. Heating of a substance results in_______
Correct
Explanation
Upon heating, particle arrangement within the state of matter gets disturbed. The disturbance is
seen either as expansion or contraction.Incorrect
Explanation
Upon heating, particle arrangement within the state of matter gets disturbed. The disturbance is
seen either as expansion or contraction. -
Question 6 of 54
6. Question
6. Which of the following remains the same when an object is cooled?
Correct
Explanation
When heated or cooled, the object may expand or contract based on the nature of the object, but the
mass remains the same. That is, the number of particles that was inside the object does not undergo
any change, only the arrangement of the particle changes.Incorrect
Explanation
When heated or cooled, the object may expand or contract based on the nature of the object, but the
mass remains the same. That is, the number of particles that was inside the object does not undergo
any change, only the arrangement of the particle changes. -
Question 7 of 54
7. Question
7. When a glass of water is heated, its volume______
Correct
Explanation
When a glass of water is heated, its volume increases and in case a glass of water is cooled its volume decreases.Incorrect
Explanation
When a glass of water is heated, its volume increases and in case a glass of water is cooled its volume decreases. -
Question 8 of 54
8. Question
8. Which of the following statement about solid is correct?
1) Particles are very close together
2) Particles are not arranged in a fixed regular pattern
3) Particles can vibrate about their fixed positionsCorrect
Explanation
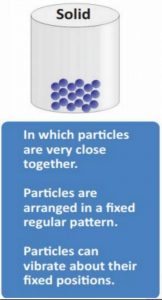 Incorrect
Incorrect
Explanation

-
Question 9 of 54
9. Question
9. Which of the following statement about liquid is correct?
1) Particles are apart from each other
2) Particles are not arranged in a fixed regular pattern
3) Particles are able to slide past one another.Correct
Explanation
 Incorrect
Incorrect
Explanation

-
Question 10 of 54
10. Question
10. Which of the following statement about gas is correct?
1) Particles are apart from each other
2) Particles are not arranged in a fixed regular pattern
3) Particles move freely over long distances.Correct
Explanation
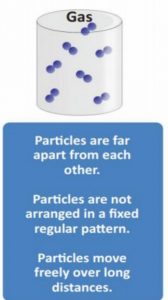 Incorrect
Incorrect
Explanation

-
Question 11 of 54
11. Question
11. Which of the following are the effects of heat gain?
1) May result in Melting, Boiling, Freezing and Condensation
2) Brings change in stateCorrect
Explanation
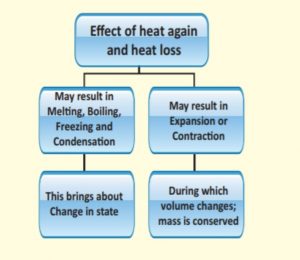 Incorrect
Incorrect
Explanation

-
Question 12 of 54
12. Question
12. Which of the following statement is correct?
1) Physical changes are the changes in which only physical properties of a substance undergo
a change
2) There is no new substance formed in a physical changeCorrect
Explanation
Physical changes are the changes in which only physical properties of a substance undergo
a change and there is no change in its chemical composition. There is no new substance formed in
a physical change.Incorrect
Explanation
Physical changes are the changes in which only physical properties of a substance undergo
a change and there is no change in its chemical composition. There is no new substance formed in
a physical change. -
Question 13 of 54
13. Question
13. Which of the following are physical properties?
1) Lustre
2) Ductility
3) Viscosity
4) DensityCorrect
Explanation
Physical properties include lustre, malleability (flexibility), and ductility (ability to be drawn into a
thin wire), density, viscosity, solubility, mass, volume and so on. Any change in these physical
properties is referred to as a physical change.Incorrect
Explanation
Physical properties include lustre, malleability (flexibility), and ductility (ability to be drawn into a
thin wire), density, viscosity, solubility, mass, volume and so on. Any change in these physical
properties is referred to as a physical change. -
Question 14 of 54
14. Question
14. Assertion(A): when a rubber band is stretched, it elongates
Reason(R): Stretching of rubber band is chemical changeCorrect
Explanation
Any change in these physical properties is referred to as a physical change. For example, when a
rubber band is stretched, it elongates. However, when then stretching is stopped, the rubber band
comes back to its original state and shape. In this example, there is no new substance formed but
the rubber band remains the same before and after elongation.Incorrect
Explanation
Any change in these physical properties is referred to as a physical change. For example, when a
rubber band is stretched, it elongates. However, when then stretching is stopped, the rubber band
comes back to its original state and shape. In this example, there is no new substance formed but
the rubber band remains the same before and after elongation. -
Question 15 of 54
15. Question
15. Which of the following statement is correct?
1) During a physical change, no new substances are formed
2) A physical change is usually temporary and reversible in nature
3) In a physical change, the chemical properties of a substance do not changeCorrect
Explanation
A physical change has following characteristics:
During a physical change, no new substances are formed. In a physical change, the chemical
properties of a substance do not change. For example, when ice cube melts, water is formed.
In this change, there is no new substance, but water is same both in ice and in water
A physical change is usually temporary and reversible in nature. For example, when water
is heated, water vapours are formed, once water vapours are cooled, water can be obtained
again.
In a physical change, the chemical properties of a substance do not change. For example,
when a piece of gold is melted, its chemical composition remains the same in the solid form
and also in the liquid form.Incorrect
Explanation
A physical change has following characteristics:
During a physical change, no new substances are formed. In a physical change, the chemical
properties of a substance do not change. For example, when ice cube melts, water is formed.
In this change, there is no new substance, but water is same both in ice and in water
A physical change is usually temporary and reversible in nature. For example, when water
is heated, water vapours are formed, once water vapours are cooled, water can be obtained
again.
In a physical change, the chemical properties of a substance do not change. For example,
when a piece of gold is melted, its chemical composition remains the same in the solid form
and also in the liquid form. -
Question 16 of 54
16. Question
16. Which of the following are examples of physical change?
1) Cutting of vegetables
2) Rusting of Iron
3) Inflating a balloonCorrect
Explanation
In a physical change, the physical properties such as colour, shape and size of a substance may
undergo a change. For example, cutting of vegetables and inflating a balloon are some examples of
physical changes in which size and shape of a substance undergoes a change. we know it is notIncorrect
Explanation
In a physical change, the physical properties such as colour, shape and size of a substance may
undergo a change. For example, cutting of vegetables and inflating a balloon are some examples of
physical changes in which size and shape of a substance undergoes a change. we know it is not -
Question 17 of 54
17. Question
17. Match the following:
I. Solid → to Liquid 1. Sublimation
II. Liquid → to Solid 2. Melting
III. Solid → to Gas 3. Freezing
IV. Gas → to Liquid 4. CondensationCorrect
Explanation
The following are some of the changes of state:
from Solid → to Liquid is Melting
from Liquid → to Gas is Vaporization
from Liquid → to Solid is Freezing
from Gas → to Liquid is Condensation
from Solid → to Gas is SublimationIncorrect
Explanation
The following are some of the changes of state:
from Solid → to Liquid is Melting
from Liquid → to Gas is Vaporization
from Liquid → to Solid is Freezing
from Gas → to Liquid is Condensation
from Solid → to Gas is Sublimation -
Question 18 of 54
18. Question
18. In an endothermic process, the speed of the molecules____
Correct
Explanation
In an endothermic process, heat is absorbed and the speed of the molecules is increased hence
they move faster.Incorrect
Explanation
In an endothermic process, heat is absorbed and the speed of the molecules is increased hence
they move faster. -
Question 19 of 54
19. Question
19. Which of the following process are endothermic process?
1) Melting
2) Freezing
3) Vaporization
4) SublimationCorrect
Explanation
Melting, vaporization, and sublimation occur when heated and hence it is called as endothermic process.Incorrect
Explanation
Melting, vaporization, and sublimation occur when heated and hence it is called as endothermic process. -
Question 20 of 54
20. Question
20. Which of the following statement about exothermic process is correct?
1) Heat is removed
2) It decreases the speed of molecules
3) Freezing and condensation are exothermic processCorrect
Explanation
In contrast, such as in freezing and condensation, heat is removed, resulting in the decreasing the
speed of the molecules causing them move slower. Such processes are called as exothermic
processIncorrect
Explanation
In contrast, such as in freezing and condensation, heat is removed, resulting in the decreasing the
speed of the molecules causing them move slower. Such processes are called as exothermic
process -
Question 21 of 54
21. Question
21. How many types of vaporization are there?
Correct
Explanation
When you put a wet cloth to dry, the water evaporates into air, leaving the clothes dry. That is there
are two types of vaporization: boiling and evaporation, the first one is by heating and the second
type of vaporization is natural.Incorrect
Explanation
When you put a wet cloth to dry, the water evaporates into air, leaving the clothes dry. That is there
are two types of vaporization: boiling and evaporation, the first one is by heating and the second
type of vaporization is natural. -
Question 22 of 54
22. Question
22. Which of the following statement is correct?
1) Boiling is a physical change
2) Boiling is the process of conversion of a liquid into vapours on heatingCorrect
Explanation
Boiling is the process of conversion of a liquid into vapours on heating. In gaseous state, only the
arrangement of molecules changes, there is no change in their chemical composition. So, boiling
is a physical change.Incorrect
Explanation
Boiling is the process of conversion of a liquid into vapours on heating. In gaseous state, only the
arrangement of molecules changes, there is no change in their chemical composition. So, boiling
is a physical change. -
Question 23 of 54
23. Question
23. Which of the following statement is correct?
1) Upon heating a liquid, the particles gain energy and vibrate more vigorously.
2) When liquid water is heated to 100ºC, it boils to become steam
3) Boiling occurs when the boiling point is reachedCorrect
Explanation
Upon heating a liquid, the particles gain energy and vibrate more vigorously. When the particles
possess enough energy, they overcome the strong forces of attraction between one another. The
particles break free from one another and move randomly. For example, when liquid water is
heated to 100ºC, it boils to become steam. Boiling occurs when the boiling point is reached. The
liquid changes to its gaseous state.Incorrect
Explanation
Upon heating a liquid, the particles gain energy and vibrate more vigorously. When the particles
possess enough energy, they overcome the strong forces of attraction between one another. The
particles break free from one another and move randomly. For example, when liquid water is
heated to 100ºC, it boils to become steam. Boiling occurs when the boiling point is reached. The
liquid changes to its gaseous state. -
Question 24 of 54
24. Question
24. Which of the following statement is correct?
1) Evaporation is the technique used to separate dissolved solids from a solid-liquid mixture.
2) This is the technique used to extract salt from sea water in salt pansCorrect
Explanation
Evaporation is the technique used to separate dissolved solids from a solid-liquid mixture. This is
the technique used to extract salt from sea water in salt pans.Incorrect
Explanation
Evaporation is the technique used to separate dissolved solids from a solid-liquid mixture. This is
the technique used to extract salt from sea water in salt pans. -
Question 25 of 54
25. Question
25. Assertion(A): Salt is formed by the evaporation of sea water
Reason(R): Evaporation makes use of the fact that the solvent in a solution can vapourise at any
temperature, leaving behind a residue of the solid that was dissolved in the liquid.Correct
Explanation
Salt is formed by the evaporation of sea water. Shallow level of sea water is impounded. Slowly the
water evaporates due to action of Sun. Ultimately salt deposits over the ground we can understand.
Evaporation makes use of the fact that the solvent in a solution can vapourise at any temperature,
leaving behind a residue of the solid that was dissolved in the liquid.Incorrect
Explanation
Salt is formed by the evaporation of sea water. Shallow level of sea water is impounded. Slowly the
water evaporates due to action of Sun. Ultimately salt deposits over the ground we can understand.
Evaporation makes use of the fact that the solvent in a solution can vapourise at any temperature,
leaving behind a residue of the solid that was dissolved in the liquid. -
Question 26 of 54
26. Question
26. Evaporation occurs at_____ of liquid
Correct
Explanation
From drying clothes to drying fish, evaporation is used. Evaporation is a slow process and occurs
only at the surface of the liquid.Incorrect
Explanation
From drying clothes to drying fish, evaporation is used. Evaporation is a slow process and occurs
only at the surface of the liquid. -
Question 27 of 54
27. Question
27. Upon cooling a liquid, the particles______ energy
Correct
Explanation
Upon cooling a liquid, the particles loose energy and vibrate less vigorously. When the particles
possess less energy, they can experience strong forces of attraction between one another. The
particles move closer to each other and movement of particles is also restricted.Incorrect
Explanation
Upon cooling a liquid, the particles loose energy and vibrate less vigorously. When the particles
possess less energy, they can experience strong forces of attraction between one another. The
particles move closer to each other and movement of particles is also restricted. -
Question 28 of 54
28. Question
28. At what temperature liquid water freezes to become ice?
Correct
Explanation
When liquid water is cooled to 0º C, it freezes to become ice. Freezing occurs when the freezing
point is reached. The liquid changes to its solid state.Incorrect
Explanation
When liquid water is cooled to 0º C, it freezes to become ice. Freezing occurs when the freezing
point is reached. The liquid changes to its solid state. -
Question 29 of 54
29. Question
29. Which of the following statement is correct?
1) Condensation is the conversion of gas into its liquid state
2) Condensation is neither physical nor chemical change
3) During this process, only the arrangement of molecules changes from the gaseous state to
liquid stateCorrect
Explanation
Condensation is the conversion of gas into its liquid state. The liquid obtained after condensation
can be converted back into gas on heating. So, condensation is also a physical process. During this
process, only the arrangement of molecules changes from the gaseous state to liquid state. So,
condensation is a physical change.Incorrect
Explanation
Condensation is the conversion of gas into its liquid state. The liquid obtained after condensation
can be converted back into gas on heating. So, condensation is also a physical process. During this
process, only the arrangement of molecules changes from the gaseous state to liquid state. So,
condensation is a physical change. -
Question 30 of 54
30. Question
30. Which of the following statement is incorrect?
1) Evaporation is the changing of a gas into its liquid state
2) Condensation is the changing of a liquid into its gas stateCorrect
Explanation
Condensation is the changing of a gas into its liquid state and it happens by cooling, whereas
Evaporation is the changing of a liquid into its gas state and it happens by heating.Incorrect
Explanation
Condensation is the changing of a gas into its liquid state and it happens by cooling, whereas
Evaporation is the changing of a liquid into its gas state and it happens by heating. -
Question 31 of 54
31. Question
31. Which of the following statement is incorrect?
1) Conversion of solid directly into gas is called sublimation.
2) There are certain solid substances like camphor, naphthalene that get converted into gas
directly upon heating without becoming liquidCorrect
Explanation
There are certain solid substances like camphor, naphthalene that get converted into gas directly
upon heating without becoming liquid. This process in which a solid is converted directly into gas
is called sublimation. In each of the above said processes, there is a change of state due to change
in temperature. But there is no change in chemical composition.Incorrect
Explanation
There are certain solid substances like camphor, naphthalene that get converted into gas directly
upon heating without becoming liquid. This process in which a solid is converted directly into gas
is called sublimation. In each of the above said processes, there is a change of state due to change
in temperature. But there is no change in chemical composition. -
Question 32 of 54
32. Question
32. Which of the following statement is correct?
1) Crystallization is a method of separation as well as a method of purification.
2) Large crystals of pure substances can be obtained from their solutions by the process of
crystallization.Correct
Explanation
Crystallization is also a special form of physical change. Large crystals of pure substances can be
obtained from their solutions by the process of crystallization. Crystallization is a method of
separation as well as a method of purification.Incorrect
Explanation
Crystallization is also a special form of physical change. Large crystals of pure substances can be
obtained from their solutions by the process of crystallization. Crystallization is a method of
separation as well as a method of purification. -
Question 33 of 54
33. Question
33. Which of the following results in formation of new substance?
Correct
Explanation
Changes that occur with the formation of new substance with different chemical composition or
transformation of a substance into another substance with the evolution or absorption of heat or
light energy are termed as chemical changes.Incorrect
Explanation
Changes that occur with the formation of new substance with different chemical composition or
transformation of a substance into another substance with the evolution or absorption of heat or
light energy are termed as chemical changes. -
Question 34 of 54
34. Question
34. Which of the following are examples of chemical change?
1) Curdling of milk
2) Burning
3) FermentationCorrect
Explanation
Chemical changes are very important in our lives. All the new substances which we use in various
fields of our life are produced as a result of chemical reactions. Some of the examples of the
importance of chemical changes are given below: Rusting of iron, burning, curdling of milk,
reaction of baking soda with lemon juice, fermentation are some examples of chemical changes.Incorrect
Explanation
Chemical changes are very important in our lives. All the new substances which we use in various
fields of our life are produced as a result of chemical reactions. Some of the examples of the
importance of chemical changes are given below: Rusting of iron, burning, curdling of milk,
reaction of baking soda with lemon juice, fermentation are some examples of chemical changes. -
Question 35 of 54
35. Question
35. Which of the following are accompanied by chemical changes?
1) Heat
2) Sound
3) GasCorrect
Explanation
In addition to new products, the following may also accompany a chemical change:
Heat, light or any other radiation may be given off or absorbed.
Sound may be produced
A change in smell may take place (or) a new smell may be given off.
A colour change may take place.
A gas may be formed.Incorrect
Explanation
In addition to new products, the following may also accompany a chemical change:
Heat, light or any other radiation may be given off or absorbed.
Sound may be produced
A change in smell may take place (or) a new smell may be given off.
A colour change may take place.
A gas may be formed. -
Question 36 of 54
36. Question
36. Explosion of a firework is a______
Correct
Explanation
Explosion of a firework is a chemical change. We know that such an explosion produces heat, light,
sound and unpleasant gases that pollute the atmosphere. That is why we are advised not to play
with fireworks.Incorrect
Explanation
Explosion of a firework is a chemical change. We know that such an explosion produces heat, light,
sound and unpleasant gases that pollute the atmosphere. That is why we are advised not to play
with fireworks. -
Question 37 of 54
37. Question
37. When slice of apple is kept in air, it becomes_____ in colour
Correct
Explanation
You must have noticed that a slice of an apple acquires a brown colour if it is not consumed
immediately. Colour of the potato remains the same when stored in water but there is change in
colour with the piece kept in air. Look at the cut brinjal kept in air.Incorrect
Explanation
You must have noticed that a slice of an apple acquires a brown colour if it is not consumed
immediately. Colour of the potato remains the same when stored in water but there is change in
colour with the piece kept in air. Look at the cut brinjal kept in air. -
Question 38 of 54
38. Question
38. The iron pillar which has not been rusted is located in________
Correct
Explanation
There is an iron pillar at the Qutub complex in Delhi which is more than 1600 years age. Even after
such a long period, the iron pillar kept in open spaces has not rusted at all. This shows that Indian
scientists made great advances in metal making technology even at 16th century which enabled
them to make this iron pillar having the quality of great rust resistance.Incorrect
Explanation
There is an iron pillar at the Qutub complex in Delhi which is more than 1600 years age. Even after
such a long period, the iron pillar kept in open spaces has not rusted at all. This shows that Indian
scientists made great advances in metal making technology even at 16th century which enabled
them to make this iron pillar having the quality of great rust resistance. -
Question 39 of 54
39. Question
39. iron +___ +____ →rust
Correct
Explanation
iron + oxygen + water →rust
2Fe + 2O2 from air + 2H2O 2Fe2O3. H2OIncorrect
Explanation
iron + oxygen + water →rust
2Fe + 2O2 from air + 2H2O 2Fe2O3. H2O -
Question 40 of 54
40. Question
40. Which of the following can be used for galvanization?
1) Zinc
2) Iron
3) Helium
4) ChromiumCorrect
Explanation
Another way of preventing rusting is to deposit a layer of a metal like chromium or zinc on iron.
This is called galvanization. Iron articles can be prevented from making contact with oxygen,
water/water vapour. A simple way is to apply a coat of paint or grease. These coats should be
applied regularly to prevent rusting.Incorrect
Explanation
Another way of preventing rusting is to deposit a layer of a metal like chromium or zinc on iron.
This is called galvanization. Iron articles can be prevented from making contact with oxygen,
water/water vapour. A simple way is to apply a coat of paint or grease. These coats should be
applied regularly to prevent rusting. -
Question 41 of 54
41. Question
41. Which of the following substances are formed during burning a paper?
1) NO2
2) carbon-dioxide
3) water vapour
4) SmokeCorrect
Explanation
Burning a piece of paper gives entirely new substances such as carbon-dioxide, water, water
vapour, smoke and ash. Heat and light are also given out during the burning of paper. We cannot
combine the products of burning of paper to form the original paper again. So, it is a permanent
change.Incorrect
Explanation
Burning a piece of paper gives entirely new substances such as carbon-dioxide, water, water
vapour, smoke and ash. Heat and light are also given out during the burning of paper. We cannot
combine the products of burning of paper to form the original paper again. So, it is a permanent
change. -
Question 42 of 54
42. Question
42. When magnesium ribbon is burnt it produce_______ coloured light
Correct
Explanation
You can see that the magnesium ribbon starts burning with a dazzling white light. Hold the burning
magnesium ribbon over a watch glass so that the powdery ash being formed by the burning of
magnesium collects in the watch glass.Incorrect
Explanation
You can see that the magnesium ribbon starts burning with a dazzling white light. Hold the burning
magnesium ribbon over a watch glass so that the powdery ash being formed by the burning of
magnesium collects in the watch glass. -
Question 43 of 54
43. Question
43. 2Mg + O2 ->
Correct
Explanation
When magnesium ribbon burns in air, then the magnesium metal combines with the oxygen of air
to form a new substance called magnesium oxide.
Magnesium + Oxygen -> Magnesium oxide
2Mg + O2 -> 2MgOIncorrect
Explanation
When magnesium ribbon burns in air, then the magnesium metal combines with the oxygen of air
to form a new substance called magnesium oxide.
Magnesium + Oxygen -> Magnesium oxide
2Mg + O2 -> 2MgO -
Question 44 of 54
44. Question
44. Which of the following statement is correct?
1) Curdling is a process in which liquid gradually turns into solid, forming clumps along the
way.
2) It is a Physical change
3) Curdling of milk is an example of irreversible changeCorrect
Explanation
We know that curdling of milk is an example of irreversible change since we cannot get back the
milk after curdling occurs. It is also called as a chemical change. Curdling is a process in which
liquid gradually turns into solid, forming clumps along the way.Incorrect
Explanation
We know that curdling of milk is an example of irreversible change since we cannot get back the
milk after curdling occurs. It is also called as a chemical change. Curdling is a process in which
liquid gradually turns into solid, forming clumps along the way. -
Question 45 of 54
45. Question
45. Which of the following breaks the sugar solution into alcohol and carbon-di-oxide?
Correct
Explanation
Fermentation is the process in which microorganisms such as yeast and certain bacteria break
down sugar solution into alcohol and carbon-di-oxide. It is an irreversible process as the alcohol
formed cannot be turned back into sugarIncorrect
Explanation
Fermentation is the process in which microorganisms such as yeast and certain bacteria break
down sugar solution into alcohol and carbon-di-oxide. It is an irreversible process as the alcohol
formed cannot be turned back into sugar -
Question 46 of 54
46. Question
46. Who was the first person to describe the process of fermentation?
Correct
Explanation
Louis Pasteur (1822- 1895), a French chemist and microbiologist was the first person to describe the
process of fermentation.Incorrect
Explanation
Louis Pasteur (1822- 1895), a French chemist and microbiologist was the first person to describe the
process of fermentation. -
Question 47 of 54
47. Question
47. Who discovered the cure for rabies?
Correct
Explanation
Louis Pasteur described that fermentation occurs in the absence of air and in the presence of
micro-organisms such as yeast. He discovered the cure for rabies.Incorrect
Explanation
Louis Pasteur described that fermentation occurs in the absence of air and in the presence of
micro-organisms such as yeast. He discovered the cure for rabies. -
Question 48 of 54
48. Question
48. Which of the following is formed when Baking soda is mixed with lemon juice?
1) Sodium citrate
2) Carbon di-oxide
3) Carbon Mono-oxide
4) WaterCorrect
Explanation
Baking soda is sodium hydrogen carbonate and lemon juice contains citric acid. So, when these
two substances are mixed together, then a chemical change takes place between sodium hydrogen
carbonate and citric acid to form three new substances: sodium citrate, carbon di-oxide and water.Incorrect
Explanation
Baking soda is sodium hydrogen carbonate and lemon juice contains citric acid. So, when these
two substances are mixed together, then a chemical change takes place between sodium hydrogen
carbonate and citric acid to form three new substances: sodium citrate, carbon di-oxide and water. -
Question 49 of 54
49. Question
49. Assertion(A): When lemon juice is mixed with soda water, they produce brisk effervescence
Reason(R): Change in pressure may also bring about a chemical changeCorrect
Explanation
We know that firing of crackers is a chemical change. Some crackers explode only when thrown
against a wall or struck with a hard substance. Thus, we could see that change in pressure may
also bring about a chemical change. When lemon juice is mixed with soda water, they produce
brisk effervescence which is otherwise not possible when they are separate.Incorrect
Explanation
We know that firing of crackers is a chemical change. Some crackers explode only when thrown
against a wall or struck with a hard substance. Thus, we could see that change in pressure may
also bring about a chemical change. When lemon juice is mixed with soda water, they produce
brisk effervescence which is otherwise not possible when they are separate. -
Question 50 of 54
50. Question
50. Assertion(A): yeast acts as the catalyst in the fermentation of sugar
Reason(R): Catalysts are substances that speed up the process of a chemical change and it
will not undergo any change during the course of the reactionCorrect
Explanation
Catalysts are substances that speed up the process of a chemical change and it will not undergo
any change during the course of the reaction. For example, yeast acts as the catalyst in the
fermentation of sugar. You will learn more about catalyst in your higher classes.Incorrect
Explanation
Catalysts are substances that speed up the process of a chemical change and it will not undergo
any change during the course of the reaction. For example, yeast acts as the catalyst in the
fermentation of sugar. You will learn more about catalyst in your higher classes. -
Question 51 of 54
51. Question
51. Burning of wood is an example of_______ change
Correct
Explanation
We saw that the burning of magnesium ribbon gives out heat and light. Similarly, burning of wood
also releases heat and light. Such changes in which heat is released are known as exothermic
changes.Incorrect
Explanation
We saw that the burning of magnesium ribbon gives out heat and light. Similarly, burning of wood
also releases heat and light. Such changes in which heat is released are known as exothermic
changes. -
Question 52 of 54
52. Question
52. Dissolution of glucose in water is________ change
Correct
Explanation
There are some changes in which heat is absorbed. For example, water absorbs heat when it
evaporates to form water vapours. Similarly, ice absorbs heat when it melts to form water. Such
changes in which heat is absorbed are known as endothermic changes. Dissolution of glucose in
water is also an endothermic change.Incorrect
Explanation
There are some changes in which heat is absorbed. For example, water absorbs heat when it
evaporates to form water vapours. Similarly, ice absorbs heat when it melts to form water. Such
changes in which heat is absorbed are known as endothermic changes. Dissolution of glucose in
water is also an endothermic change. -
Question 53 of 54
53. Question
53. Which of the following are periodic change?
1) Motion of the seconds-hand
2) Rotation of earth
3) Beating of the heartCorrect
Explanation
Changes that repeat themselves after a definite interval of time are called periodic changes.
Rotation and Revolution of earth, beating of the heart, clock striking every hour, motion of the
seconds-hand / minute-hand / hour-hand of a clock are some examples of periodic changes.Incorrect
Explanation
Changes that repeat themselves after a definite interval of time are called periodic changes.
Rotation and Revolution of earth, beating of the heart, clock striking every hour, motion of the
seconds-hand / minute-hand / hour-hand of a clock are some examples of periodic changes. -
Question 54 of 54
54. Question
54. Which of the following are Non-periodic changes?
1) Eruption of a volcano
2) Streak of lighting flash across the sky
3) Seasonal changeCorrect
Explanation
Changes that do not repeat themselves after a definite interval of time and occur randomly are
called non-periodic changes. Eruption of a volcano, occurrence of an earthquake, a streak of
lighting flash across the sky during a thunderstorm, running of a batsman between the wickets,
movement of legs while dancing are a few examples of non-periodic changes.Incorrect
Explanation
Changes that do not repeat themselves after a definite interval of time and occur randomly are
called non-periodic changes. Eruption of a volcano, occurrence of an earthquake, a streak of
lighting flash across the sky during a thunderstorm, running of a batsman between the wickets,
movement of legs while dancing are a few examples of non-periodic changes.
Leaderboard: Changes Around Us Online Test 7th Science Lesson 9 Questions in English
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||