Air Notes 8th Science Lesson 11 Notes in English
8th Science Lesson 11 Notes in English
11] Air
Introduction:
Air is a mixture of gases that surrounds our planet earth. It is essential for the survival of all the living things. Air contains 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide and small amount of other gases. We breathe in oxygen and breathe out carbon dioxide. Plants in turn use carbon dioxide for photosynthesis and release oxygen into the atmosphere. Since men have been cutting down trees for their needs, the amount of carbon dioxide in the atmosphere is increasing. This is responsible for the raising of atmospheric temperature. Industries and vehicles release gases like carbon monoxide and sulphur dioxide into the atmosphere. This has resulted in effects like global warming and acid rain which affect us in many ways. In total, the quality of air is gone in the modern days. In this lesson we are going to study about the effects like green house effect, global warming and acid rain. We will also study about occurrence and properties of the gases oxygen, nitrogen and carbon dioxide.
Oxygen:
All living things in the world need oxygen. We cannot imagine the world without oxygen. Swedish chemist C.W. Scheele first discovered oxygen in 1772. He called the gas fire air or vital life because it was found to support the process of burning. It was independently discovered by the British scientist Joseph Priestley in 1774. Lavoisier named oxygen. The name oxygen comes from the Greek word ‘oxygenes’ which means ‘acid producer’. It is called so because early chemists thought that oxygen is necessary for producing acids.
Occurrence of Oxygen:
Oxygen is the most abundant element on the earth by mass and the third most abundant element after Hydrogen and Helium in the universe. It occurs both in free state and combined state. It is present in free state as diatomic molecule (O2) in the atmosphere. Most of this has been produced by photosynthesis in which the chlorophyll present in the leaves of plants uses solar energy to produce glucose.
Percentage of Oxygen
| Oxygen in free state | Oxygen in combined state | ||
| Source | Percentage | Source | Percentage |
| Atmospheric air | 21 % | Plants and animals | 60 – 70 % |
| Water | 88 – 90 % | Minerals in the form of silicates, carbonates and oxides | 45 – 50 % |

In combined state it is present in the earth’s crust as silicates and metal oxides. It is also found in water on the surface of the earth. Tri atomic molecule (O3) known as ozone is present in the upper layers of the atmosphere.

Percentage of elements in the Earth’s crust
Physical properties of Oxygen:
- Oxygen is a colourless, odourless and tasteless gas.
- It is a poor conductor of heat and electricity.
- Oxygen dissolves readily in cold water.
- It is denser than air.
- It can be made into liquid (liquified) at high pressure and low temperature.
- It supports combustion.
Chemical properties of Oxygen:
- Combustibility:
Oxygen is a non-combustible gas as it does not burn on its own. But, it supports the combustion of other substances.
- Reaction with metals:
Oxygen reacts with metals like sodium, potassium, magnesium, aluminium, iron etc., to form their corresponding metal oxides which are generally basic in nature. But the metals differ in their reactivity towards oxygen.
Metal + Oxygen ???? Metal oxide
Example:
4Na + O2 ???? 2Na2O
Sodium Oxygen Sodium oxide
Reactivity of Oxygen with metals
| Metal | Temperature | Product formed |
| K | Room temperature | Potassium Oxide (K2O) |
| Mg | Heating slightly | Magnesium Oxide (MgO) |
| Ca | Heating slightly | Calcium Oxide (CaO) |
| Fe
Cu Ag |
High temperature | Iron Oxide (Fe3O4)
Cupric Oxide (CuO) Silver Oxide (Ag2O) |
| Au
Pt |
Even at high temperature | No action |
- Reaction with non metals:
Oxygen reacts with various non-metals like hydrogen, nitrogen, carbon, sulphur, phosphorus etc., to give corresponding non metallic oxides, which are generally acidic in nature.
Non-metal + Oxygen ???? Non-metallic oxide
Example:
C + O2 ???? CO2
Carbon Oxygen Carbon dioxide
Reaction of Oxygen with non metals
| Non metal | Products formed |
| C | Carbon dioxide (CO2) |
| N | Nitric oxide (NO) |
| S | Sulphur dioxide (SO2) |
| P | Phosphorus trioxide (P2O3) or Phosphorus pentoxide (P2O5) |
- Reaction with Hydrocarbons:
Hydrocarbons (compounds containing C and H) react with oxygen to form carbon dioxide and water vapour. E.g. Wood, Petrol, Diesel, LPG, etc. When they burn in oxygen, they produce heat and light energy. Hence they serve as fuel.
Hydrocarbon + O2 CO2 + Water vapour + Heat energy + Light
- Rusting:
The process of conversion of iron into its hydrated form of oxide in the presence of air and moisture (humid atmosphere) is called rusting. Rust is hydrated ferric oxide.
4Fe + 3O2 ???? 2Fe2O3
Fe2O3 + x H2O ???? Fe2O3 • x H2O
(rust)
(x is the number of water molecules which is variable)
Uses of Oxygen:
- It is used as oxy-acetylene cylinder for cutting and welding metals.
- It is used to remove carbon impurities from steel.

Uses of Oxygen
- Plants and animals use oxygen from the air for respiration.
- It is used as rocket fuel.
- It is used for artificial respiration by scuba divers, mountaineers, astronauts, patients etc.
- Mixed with powdered charcoal it is used as explosives.
- It is used in the synthesis of methanol and ammonia.
Nitrogen:
Nitrogen is one of the most important elements. Animals and plants need nitrogen for their growth. All living organisms (including us) contain nitrogen. It is an essential element present in proteins and nucleic acids which are the ‘building blocks’ of all living things. It was first isolated from the air by Daniel Rutherford in 1772. The name ‘nitrogen’ is derived from the Greek words ‘nitron’ and ‘gene’ meaning ‘I produce nitre’. Nitre is potassium nitrate compound of nitrogen. Antoine Lavoisier suggested the name azote, from the Greek word meaning ‘no life’.
Occurrence of Nitrogen:
Nitrogen is the fourth most abundant element in the human body. It accounts for about three percent of the mass of the human body. It is thought to be the seventh most abundant element in the universe. Titan, the largest moon of Saturn, has an atmosphere made up of 98% Nitrogen. Nitrogen occurs both in free state and combined state. Nitrogen exists in free state in the atmospheric air as dinitrogen (N2). It is present in volcanic
gases and gases evolved by burning of coal. Nitrogen is present in combined state in the form of minerals like nitre (KNO3) and chile salt petre (NaNO3). It is present in organic matters such as protein, enzymes, nucleic acid etc.
Physical properties of Nitrogen:
- It is a colourless, tasteless and odourless gas.
- It is slightly lighter than air.
- It is slightly soluble in water.
- Nitrogen becomes a liquid at low temperature and looks like water.
- When it freezes, it becomes a white solid.
- It is neutral to litmus like oxygen.
Chemical properties of Nitrogen:
- Chemical reactivity:
Nitrogen is inactive at ordinary conditions. It combines with many elements at high temperature and pressure or in the presence of catalyst.
- Combustion:
Nitrogen is neither combustible nor a supporter of combustion. So nitrogen in the air moderates the rate of combustion.
- Reaction with metals:
Nitrogen reacts with metals like lithium, calcium, magnesium etc., at high temperature to form their corresponding metal nitrides.
![]()
Example:
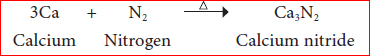
- Reaction with non metals:
Nitrogen reacts with non-metals like hydrogen, oxygen etc., at high temperature to form their corresponding nitrogen compounds.

Example:
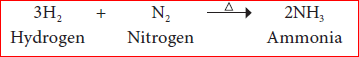
Uses of Nitrogen:
- Liquid nitrogen is used as a refrigerant.
- It provides an inert atmosphere for conducting certain chemical reactions.
- It is used to prepare ammonia (by Haber’s process) which is then converted into fertilizers and nitric acid.
- Nowadays it is used as a substitute for compressed air in tyres.
- It is used for filling the space above mercury in high temperature thermometer to reduce the evaporation of mercury.
- Many explosives such as TNT (Trinitrotoluene), nitroglycerin, and gun powder contain nitrogen.
- It is used for the preservation of foods, manufacturing of stainless steel, reducing fire hazards, and as part of the gas in incandescent light bulbs.

Uses of Nitrogen
Nitrogen fixation:
Nitrogen gets circulated in the air, soil and living things as the element itself or in the form of its compounds. Just as there is a circulation of carbon in nature so also there is a circulation of nitrogen. It is essential for the proper growth of all plants. The plants cannot make use of the elemental nitrogen from the air as such. The plants require soluble compounds of nitrogen. Thus, plants depend on other processes to supply them with nitrates. Any process that converts nitrogen in the air into a useful nitrogen compound is called nitrogen fixation. Fixation of nitrogen is carried out both naturally and by man.
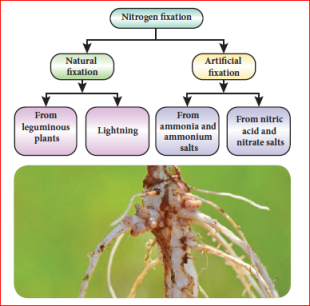
Nitrogen fixation in leguminous plants
Carbon dioxide:
Carbon dioxide is a chemical compound in which one carbon and two oxygen atoms are bonded together. It is a gas at room temperature. It is represented by the formula CO2. It is found in the earth’s atmosphere and it sends back the solar energy which is reflected by the surface of the earth, to make it possible
for living organisms to survive. When carbon dioxide accumulates more in the atmosphere it produces harmful effects.
Occurrence of Carbon dioxide:
Carbon dioxide is present in air to the extent of about 0.03% by volume. It is evolved by the plants and animals during respiration and is produced during fermentation reactions. Much of the naturally occurring CO2 is emitted from the magma through volcanoes. CO2 may also originate from the bio degradation of oil and gases. Carbon dioxide emitted by human upset the natural balance of the carbon cycle. Man-made CO2 in the atmosphere has increased global temperatures which is warming the planet. While CO2 derived from fossil-fuel is a very small component of the global carbon cycle, the extra CO2 is cumulative because the natural carbon exchange cannot absorb all the additional CO2.
Physical properties of Carbon dioxide:
- Carbon dioxide is a colourless and odourless gas.
- It is heavier than air.
- It does not support combustion.
- It is fairly soluble in water and turns blue litmus slightly red. So it is acidic in nature.
- It can easily be liquified under high pressure and can be solidified. This solid form of CO2 is called dry ice which undergoes sublimation.
Chemical properties of Carbon dioxide:
- Combustibility:
It is non-combustible gas and not a supporter of combustion.
- Reaction with metals:
Lighter metals like sodium, potassium and calcium, combine with CO2 to form corresponding carbonates whereas magnesium gives its oxide and carbon.
Example:
4Na + 3CO2 ???? 2Na2CO3 + C
Sodium Sodium carbonate
2Mg + CO2 ???? 2MgO + C
Magnesium Magnesium oxide
- Reaction with sodium hydroxide (Alkali):
Sodium hydroxide (base) is neutralized by carbon dioxide (acidic) to form sodium bicarbonate (salt) and water.
NaOH + CO2 ???? NaHCO3 + H2O
Sodium bicarbonate
- Reaction with Lime water (Calcium hydroxide):
When a limited amount of CO2 is passed through lime water, it turns milky due to the formation of insoluble calcium carbonate.
Ca(OH)2 + CO2 ???? CaCO3 + H2O
Calcium carbonate
When an excess amount of CO2 is passed through lime water, it first turns milky and the milkyness disappears due to the formation of soluble calcium hydrogen carbonate, Ca(HCO3)2.
Uses of Carbon dioxide:
- CO2 is used to prepare soft drinks or aerated drinks.
- It is used in fire extinguishers.
- It is used in the manufacturing of sodium carbonate by Solvay process.
- Solid carbon dioxide, called as dry ice is used as a refrigerant. The gas is so cold that moisture in the air condenses on it, creating a dense fog which is used in stage shows and movie effects.
- It is used along with ammonia in the manufacture of fertilizers like urea.
- CO2 can be used in the preservation of food grains, fruits etc.
Solid carbon dioxide
Green House Effect and Global Warming:
The solar radiation is absorbed by the surface of land and ocean. In turn, they release infra red radiation
or heat into the atmosphere. Certain gaseous molecules present in the atmosphere absorb the infra red rays and reradiate the heat in all directions. Hence, these gases maintain the temperature of earth’s surface. The gases which absorb these radiations are called green house gases and this effect is called green house effect.
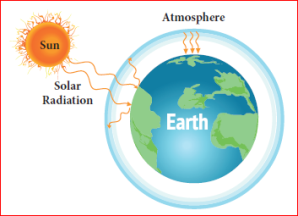
Greenhouse effect
The green house gases are CO2, N2O, CH4, CFC (Chlorofluoro carbon) etc. The increase in the levels of these gases results in the gradual increase of temperature of the earth’s surface. This green house effect is caused due to increase in the air pollutants and it results in the average increase of temperature of the atmosphere. This is called as Global warming.
Effects of Global warming:
The following are the effects of global warming.
- Melting of ice cap and glaciers.
- Increase in frequency of floods, soil erosion and unseasonal rains.
- Loss of biodiversity due to the extinction of coral reefs and other key species.
- Spreading of waterborne and insectborne diseases.
Preventive measures:
In order to save the earth and its resources we need to take certain measures. Some of the measures are given below:
- Reducing in the use of fossil fuels.
- Controlling deforestation.
- Restricting the use of CFCs.
- Planting more trees.
- Reducing, reusing and recycling resources.
- Using renewable energy resources.
Acid rain:
Rain water is actually the purest form of water. However, pollutants such as oxides of nitrogen (N2O, NO2) and sulphur (SO2, SO3) in the air released by factories, burning fossil fuels, eruption of volcanoes etc., dissolve in rain water and form nitric acid and sulphuric acid which adds up to the acidity of rain water. Greenhouse effect Hence, it results in acid rain.
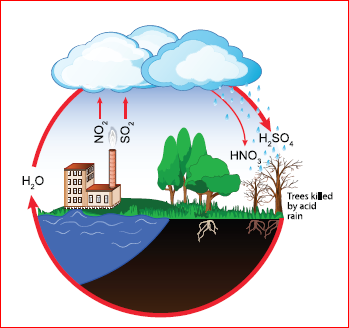
Acid Rain
Effects of Acid rain:
Acid rain affects us in many ways. Some of the consequences are given below.
- It irritates eyes and skin of human beings.
- It inhibits germination and growth of seedlings.
- It changes the fertility of the soil, destroys plants and aquatic life.
- It causes corrosion of many buildings, bridges etc.
Preventive measures:
Acid rain and its effects can be controlled by the following ways.
- Minimizing the usage of fossil fuel such as petrol, diesel etc.,
- Using CNG (Compressed Natural Gas).
- Using non-conventional source of energy.
- Proper disposal of the industrial wastes.
Points to Remember:
- Oxygen exists in nature as silicates, carbonates, oxides and water. It also exists in free state as part of air in the atmosphere.
- Oxygen is a colourless and odourless gas. It dissolves sparingly in water. It is denser than air.
- Metals like magnesium, iron and sodium burn in oxygen and give basic oxides.
- Bacteria convert atmospheric nitrogen directly into soluble nitrogen compounds.
- Though nitrogen is inactive at ordinary condition, it combines with many elements at high temperature and pressure or in the presence of catalyst.
- Carbon dioxide cannot exist as a liquid at atmospheric pressure. It occurs as carbonates in nature.
- Carbon dioxide is acidic in nature and turns lime water milky. It is used in fire extinguisher.
- Global warming refers to an average increase in the temperature of the atmosphere or simply it is the warming of the earth.
- The green house gases are carbon dioxide, methane, nitrous oxide, chlorofluoro carbons, etc.
Glossary:
Atmosphere – Gaseous jacket that surrounds the earth.
Fixation of nitrogen – Process that converts nitrogen in the air into nitrogen compounds.
Global warming – An average increase in the temperature of the atmosphere.
Green house effect – Trapping of radiation from the sun by green house gases in the atmosphere that leads to rise in the earth’s atmospheric temperature
Haber’s process – Synthesis of ammonia from nitrogen and hydrogen with the help of catalyst under 500 atm pressure and 550˚C temperature
Oxygen – A Greek word meaning ‘acid producers’ from which the name ‘Oxygen’ is derived.
Soda water – A form of water produced when carbon dioxide is dissolved in water under pressure.
Sublimation – Process of conversion of solid directly to vapour without reaching liquid state.
Do You Know?
Oxygen is about two times more soluble in water than nitrogen. If it had the same solubility as nitrogen, then less oxygen would be present in seas, lakes and rivers that will make life much more difficult for living organisms.
If oxygen has the capacity to burn itself, striking a match stick will be enough to burn all the oxygen in our planet’s atmosphere.
Now-a-days nitrogen is used as a substitute for compressed air in tyres. Have you noticed it? Why do people prefer nitrogen instead of compressed air in tyres?
The process of conversion of solid into vapour without reaching liquid state is called sublimation.
Venus’ atmosphere consists of roughly 96-97% carbon dioxide. Because of the amount of carbon dioxide present, the surface of Venus continually retains heat and as such, the surface temperature of Venus is roughly 462°C, making it the hottest planet in our solar system.
Aerated water is nothing but carbon dioxide dissolved in water under pressure. This is also called ‘soda water’.
Acid rain has pH less than 5.6 whereas pH of pure rain water is around 5.6 due to dissolution of atmospheric CO2 in it.