Air Notes 6th Science Lesson 10 Notes in English
6th Science Lesson 10 Notes in English
10] Air
Introduction
- Air is present everywhere around us. We cannot see air.
- But we can feel its presence in so many ways. For example, we feel air when the trees rustle, clothes hanging on a clothes-line sway, pages of an open book flutters when the fan is switched on, when kites fly in the sky.
- We cannot see, touch or taste air but we can feel it. It is the air that makes all these movements possible. Thus, we can understand that air is present all around us.
- Air is necessary for us to live. We can live without food for some days, without water for a few hours, but cannot survive without air for more than a few minutes. So, air is very important for all living beings to survive.
- When air is moving it is called wind. It is cool and soothing as breeze. When air moves with force it can even uproot trees and blow off the roof tops.
Atmosphere
- Our earth is surrounded by a huge envelope of air called the atmosphere.
- Atmosphere extends to more than 800km above the surface of earth and is held in place by the earth’s gravity. The atmosphere protects us from many harmful rays coming from the sun.
- The air envelope is thicker near the earth’s surface and as we go higher the density and the availability of air gradually decreases.
- This is because, as we go higher, the force of gravity decreases, so it is not able to hold large amount of air.
- The atmosphere is made of five different layers – the troposphere, the stratosphere, the mesosphere, the ionosphere and the exosphere.
- The troposphere is the layer closest to the earth. It is the layer in which we live. It extends upwards for about 16km above the surface of the earth.
- Movement of wind takes place in this layer. It also contains water vapour, which is responsible for making clouds. This layer is responsible for the weather we experience on earth.
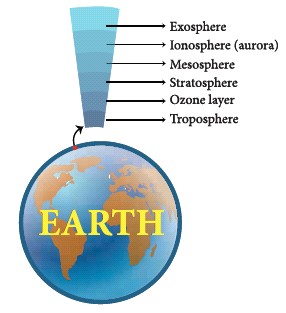
- Aircrafts usually fly above this layer to avoid strong winds and bad weather.
- The stratosphere lies above the troposphere. This layer has the ozone layer in it. The ozone layer protects all life on earth from the harmful ultraviolet rays of the sun.
Experimental verification of presence of Oxygen, Carbon-di-oxide and Nitrogen in Air
Is air a thing or a composite mixture?
- For long time, that is, until eighteenth century, human thought ‘air’ as a fundamental constituent of matter.
- However an ingenious experiment conducted by Joseph Priestley in 1774 showed that “air is not an elementary substance, but a composition,” or mixture of gases.
- He was also able to identify a colorless and highly reactive gas which was later named ‘oxygen’ by the great French chemist Antoine Lavoisier.

![]()
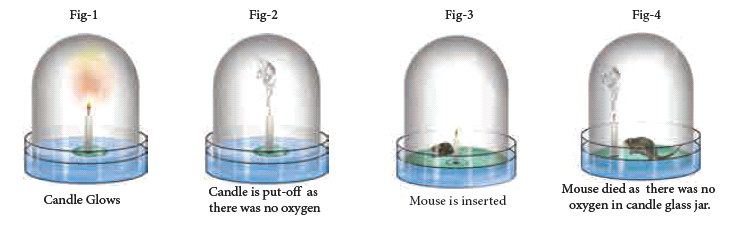
- Priestley took a tub of water and made a float and placed a candle on it. He covered the candle with a glass jar.
- [As the bottom portion of the jar was filled with water, no air can enter or exit and hence the jar was completely sealed (Fig-1)]. As you would have guessed the candle flame was extinguished in a very short time.
- He used a magnifying glass to focus the sun rays to light the candle. Thus he tried to relight the candle many times without opening the sealed jar (Fig-2). The candle could not be relit.
- It was clear that something in the air was being used for burning and being converted into another substance.
- Once the substance in the air that was aiding the burning was completely used by the burning flame and converted into another substance, the flame went out.
- [Later chemist named the substance necessary for burning as oxygen and during the process of burning oxygen is converted mostly into carbon dioxide.]
- Now as the jar was inside the water, Priestley could gently lift the jar and place a live mouse inside it without allowing outside air to enter the jar (Fig-3).
- Without oxygen, as you would have guessed, the mouse died (Fig-4). It was clear that oxygen was necessary for the survival of the mouse.
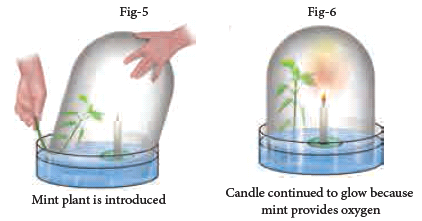
- In the next step, he gently lifted the jar and placed a mint plant (Fig-5). (Note: Look at the Figure- 5; you could see that the plant is inserted into the bell jar when the jar is very much inside the water.
- This is done to ensure that the outside air is not entering into the bell jar.) Plant being a living thing like mouse, perhaps he thought, would die.
- Instead, the plant survived. After placing the mint plant, he lit up the candle and it continued to burn (Fig-6).
- In fourth experiment, he took a jar, burned a candle and converted all oxygen into carbon dioxide.
- He placed a mint plant and a mouse into this jar. Both the plant and the mouse survived (Fig-7). He found that plants and animals have a synergy.
- Animals consume oxygen and release carbon-di-oxide and plants take up carbon dioxide and release oxygen.
- During 1730 – 1799, Jan Ingenhousz showed that sunlight is essential to the plant to carry out photosynthesis and also to purify air that is fouled by breathing animals or by burning candles.
- From these experiments it was clear that “air” was a composite mixture of many gases like oxygen and carbon- di-oxide.
Test for Carbon-di-oxide in air
- Pour some lime water in a glass tumbler.
- Bubble some air using a straw through the limewater.
- After a few minutes, look at the lime water carefully.
- The lime water will produce a white precipitate and that the lime water will eventually turn to a milky white solution. This shows the presence of carbon-di-oxide in air.

Composition of Air
- From Priestley’s experiment which was followed by Ingenhousz and Rutherford, we came to know that air was not just one substance.
- We will now describe what air is made up of. This is called composition of air.
- The major component of air is nitrogen. Almost four – fifth of air is nitrogen. The second major component of air is oxygen. About one – fifth of air is oxygen.
- In addition to nitrogen and oxygen gases, air also contains small amount of carbon–di– oxide, water vapour and some other gases like argon, helium etc. The air may also contain some dust particles.
- The composition of air in terms of percentage of its various components can be written as follows:
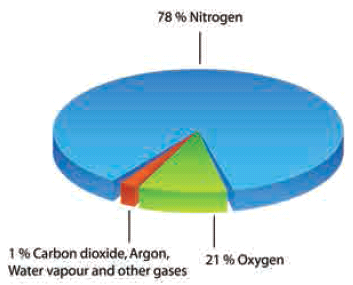
The composition of air changes slightly from place to place and also from season to season. For example,
- Air over industrial cities usually has a higher amount of carbon-di-oxide in it than the air over open spaces.
- Air in coastal areas may have more water vapour than inland areas.
- Air also contains more water vapour in rainy season.
- The amount of dust in the air is more in windy places than other areas.
Test for the presence of dust particles in air
- You might have seen the sunlight entering into a dark room through a narrow slit and making shiny dust particles dancing merrily on the path of sunlight.
- Actually, the air in a room always contains some dust particles, but they are so small that normally they are not visible to us.
- When a beam of sunlight falls on them, the tiny dust particles become visible.
- Take a graph sheet. Using marker pens draw a 5×5 cm square on the graph. Apply a thin film of grease on the graph sheet. This sheet will serve as dust collector. Make four or five graph sheets.
- Discuss in the whole class, as where to place the dust collectors, how long to collect dust particles and place the dust collectors in agreed positions.
- Ensure that the dust collectors do not get blown away.
- After the time scheduled for performing this activity is reached, remove the paper and count the number of collected dust particles in the marked area in all the sheets, using a magnifying glass at the dust collector. We can see something similar to the diagram below:-
- Then, calculate the mean number of dust particles in the marked area.
![]()
The range of the dust can also be calculated as given below:-
Range = Maximum value – minimum value
- Collect details from all the areas where we have kept the dust collector sheets.
Test for water vapour in air
- Take a few ice cubes in a glass. Keep it on the table for a few minutes. Observe what happens.
- You could see tiny droplets of water all over the outer surface of the glass. From where do these droplets come? The water vapour present in the air condenses on the cold surface of the glass. This shows that air contains water vapour.

Burning and Combustion
- When we burn a candle, paper, kerosene, and coal, wood or cooking gas (LPG), oxygen is needed.
- The oxygen needed for the burning of candle, paper, kerosene, coal, wood and cooking gas comes from the air around us.
- Thus, for burning a substance continuously so as to make fire, a continuous supply of fresh air is needed.
- If we cut off the supply of fresh air to a burning substance, then the burning substance will not get oxygen necessary for burning to continue and hence the substance will stop burning.
- In rockets, as they go high in the atmosphere, the availability of oxygen is considerably reduced. Therefore in rockets along with the fuel, oxygen is also carried for combustion.
- The process of burning of a substance in the presence of oxygen and releasing a large amount of light and heat is called burning. If the process does not emit flame then it is called combustion.
Importance of air for survival of plants and animals
Respiration in plants
- Plants require energy for their growth and hence respiration also occurs in plants.
- During respiration, plants take in oxygen and release carbon–di–oxide, just as animals do.
- Gaseous exchange with air in atmosphere takes place in plants with the help of tiny holes called stomata present on their leaves.
Photosynthesis
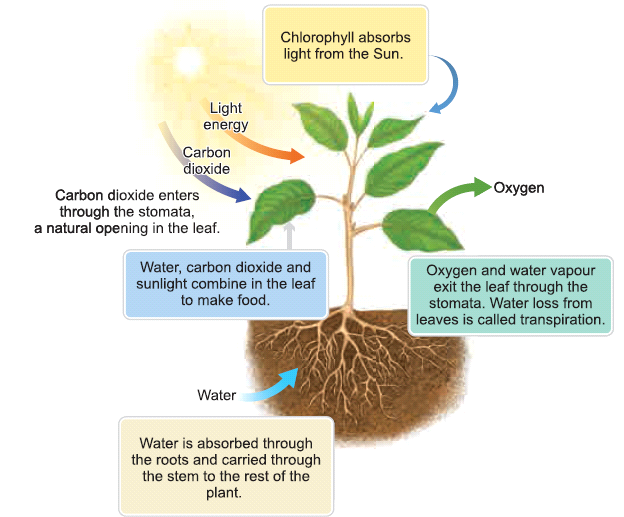
- Plants manufacture food by a process called photosynthesis.
- During photosynthesis, Carbon-di-oxide from the air and water from the soil react in the presence of sunlight to produce food.
- Most plants possess a green pigment called chlorophyll and it is also used-up in the process of photosynthesis.
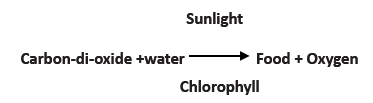
- The word equation given below explains the process of photosynthesis.
- Plants release oxygen during photosynthesis which is much more than the oxygen consumed by the plants, during respiration.
Respiration in Animals
- When we breathe in air, the oxygen present in the air reacts chemically with digested food within the body to produce carbon-di-oxide gas, water vapour and energy.
- This energy is required to carry out many processes in the body such as movement, growth and repair.
- This process by which oxygen reacts with digested food to form carbon-di-oxide, water vapour and energy is called respiration. The process can be represented by a word equation as given below:-
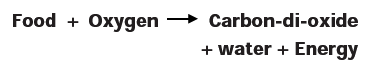
- Carbon-di-oxide formed during respiration dissolves in the blood and is exhaled out of the body through the lungs.
- The inhaled and exhaled air thus contain the same substances but in different proportion, except nitrogen which is present in the same amount.
- Inhaled air contains more oxygen while the exhaled air contains more carbon-di-oxide.
- Let us have a look at the following table to compare the composition of air in inhaled and exhaled air.
Respiration of plants and animals in water
- The water of ponds, lakes, rivers and seas has some amount of dissolved air containing oxygen in it.
- The plants and animals that live in water use the oxygen dissolved in water for breathing. For example, frogs respire through their skin, fish respire using their gills.
Uses of Air
- Air is used by plants and animals for breathing.
- Air is used for burning fuels like wood, coal, kerosene, LPG etc.
- Compressed air is used to fill tyres of various kinds of vehicles.
- Air plays an important role in maintaining the water cycle in the nature.
- Ozone layer, present in the atmosphere, helps in preventing harmful radiations of the sun from reaching the earth’s surface.
Under extra – ordinary conditions such as:
A. a patient having breathing difficulties,
B. a mountaineer climbing a high mountain,
C. a diver going deep into the sea- oxygen gas cylinders are used for breathing purposes.
- Blowing air is used to turn the blades of wind mills.
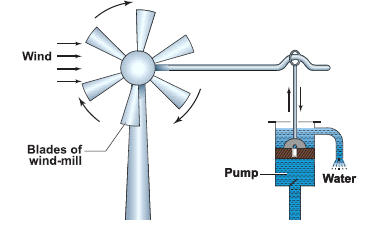
- The wind mills are used to draw water by running pumps, run flour mills and to generate electricity.
More to know:
- A weathercock shows the direction in which the air is moving at a particular place. You can also make a wind sock to find the direction of the wind. Can you try it yourself?
- Daniel Rutherford, a Scottish chemist, discovered nitrogen. He removed oxygen and converted it into carbon-di-oxide using an inverted bell jar using a burning candle.
- He passed this air without oxygen through lime water and removed carbon-di-oxide also.
- Once the carbon-di-oxide was removed in that air, neither a candle burned nor a plant breathed.
- Hence he was sure that the remaining air he had did not have oxygen and carbon-di-oxide.
- He was able to produce a gas, which showed the same property of the air without oxygen and carbon-di-oxide. Hence this gas was named ‘nitrogen’.
- When carbon-di-oxide is cooled to -570 C, it directly becomes a solid, without changing to its liquid state.
- It is called dry ice and is a good refrigerating agent.
- Dry ice is used in trucks or freight cars for refrigerating perishable items such as meat and fish while transporting them.