ACIDS, BASES AND SALTS Notes 9th Science Lesson 14 Notes in English
ACIDS, BASES AND SALTS
Introduction
- We know that the physical world around us is made of large number of chemicals.
- Soil, air, water, all the life forms and the materials that they use are all consist of chemicals.
- Out of such chemicals, acids, bases and salts are mostly used in everyday life.
- Let it be a fruit juice or a detergent or a medicine, they play a key role in our day-to- day activities.
- Our body metabolism is carried out by means of hydrochloric acid secreted in our stomach.
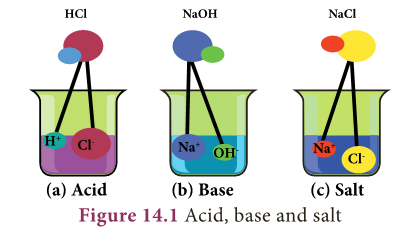
- An acid is a the compound which is capable of forming hydrogen ions (H+) in aqueous solution whereas a base is a compound that forms hydroxyl ions (OH–) in solution.
- When an acid and a base react with each other, a neutral product is formed which is called salt.
- In this lesson let us discuss about them in detail.
Acids
- Look at the pictures of some of the materials used in our daily life, given below: All these edible items taste similar i.e. sour.
- What causes them to taste sour?
- A certain type of chemical compounds present in them gives sour taste. These are called acids.
- The word ‘acid’ is derived from the Latin name “acidus” which means sour taste.
- Substances with sour taste are called acids.
- In 1884, a Swedish chemist Svante Arrhenius proposed a theory on acids and bases.
- According to Arrhenius theory, an acid is a substance which furnishes H+ ions or H3O+ ions in aqueous solution.

- They contain one or more replaceable hydrogen atoms.
- For example, when hydrogen chloride is dissolved in water, it gives H1 and Cl2 ions in water.
![]()
- What happens to an acid or a base in water? Do acids produce ions only in aqueous solution?
- Hydrogen ions in HCl are produced in the presence of water.
![]()
- The separation of H+ ion from HCl molecules cannot occur in the absence of water.
- Hydrogen ions cannot exist alone, but they exist in combined state with water molecules.
- Thus, hydrogen ions must always be H+ (or) Hydronium (H3O+).
![]()
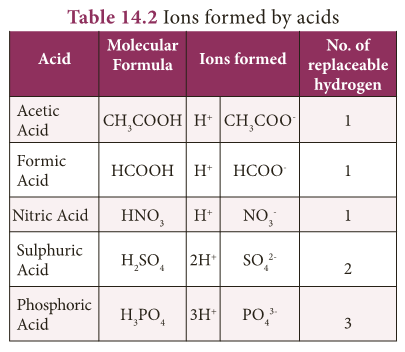
- The following table enlists various acids and the ions formed by them in water.
Classification of Acids
- Acids are classified in different ways as given below:
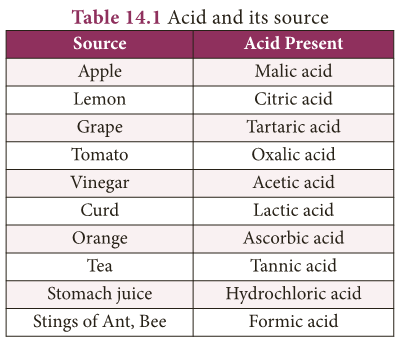
(a) Based on their sources:
- Organic Acids: Acids present in plants and animals (living things) are organic acids. Example: HCOOH, CH3COOH
- Inorganic Acids: Acids prepared from rocks and minerals are inorganic acids or mineral acids. Example: HCl, HNO3, H2SO4
(b) Based on their Basicity
- Monobasic Acid: Acid that contain only one replaceable hydrogen atom per molecule is called monobasic acid.
- It gives one hydrogen ion per molecule of the acid in solution. Example: HCl, HNO3
- Dibasic Acid: An acid which gives two hydrogen ions per molecule of the acid in solution. Example: H2SO4, H2CO3
- Tribasic Acid: An acid which gives three hydrogen ions per molecule of the acid in solution. Example: H3PO4
(c) Based on Ionisation
- Acids get ionised in water (produce H+ ions) completely or partially.
- Based on the extent of ionisation acids are classified as below.
- Strong Acids: These are acids that ionise completely in water. Example: HCl
- Weak Acids: These are acids that ionise partially in water. Example: CH3COOH.
(d) Based on Concentration
- Concentrated Acid: It has relatively large amount of acid dissolved in a solvent.
- Dilute Acid: It has relatively smaller amount of acid dissolved in solvent.
Properties of Acids
- They have sour taste.
- Their aqueous solutions conduct electricity since they contain ions.
- Acids turns blue litmus red.
- Acids react with active metals to give hydrogen gas.

- Acids react with metal carbonate and metal hydrogen carbonate to give carbon dioxide.
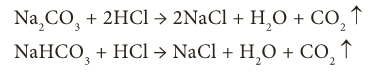
- Acids react with metallic oxides to give salt and water.
![]()
- Acids react with bases to give salt and water.
![]()
- The reaction is known as neutralisation reaction.
Activity 1
- Take about 10 ml of dilute hydrochloric acid in a test tube and add a few pieces of zinc granules into it.
- What do you observe?
- Why are bubbles formed in the solution?
- Take a burning candle near a bubble containing hydrogen gas, the flame goes off with a ‘Popping’ sound.
- This confirms that metal displaces hydrogen gas from the dilute acid.
Caution:
- Care must be taken while mixing any concentrated inorganic acid with water.
- The acid must be added slowly and carefully with constant stirring to water since it generates large amount of heat.
- If water is added to acid, the mixture splashes out of the container and it may cause burns.
Uses of Acids
- Sulphuric acid is called King of Chemicals because it is used in the preparation of many other compounds.
- It is used in car batteries also.
- Hydrochloric acid is used as a cleansing agent in toilets.
- Citric acid is used in the preparation of effervescent salts and as a food preservative.
- Nitric acid is used in the manufacture of fertilizers, dyes, paints and drugs.
- Oxalic acid is used to clean iron and manganese deposits from quartz crystals.
- It is also used as bleach for wood and removing black stains.
- Carbonic acid is used in aerated drinks.
- Tartaric acid is a constituent of baking powder.
Aquaregia
- We know that metals like gold and silver are not reactive with either HCl or HNO3. But the mixture of these two acids can dissolve gold.
- This mixture is called Aquaregia.
- It is a mixture of hydrochloric acid and nitric acid prepared optimally in a molar ratio of 3:1.
- It is a yellow-orange fuming liquid.
- It is a highly corrosive liquid, able to attack gold and other substances.
- Chemical formula: 3 HCl + HNO3
- Solubility in Water: Miscible in water
- Melting point: – 42°C (- 44°F, 231K)
- Boiling point: 108°C (226°F, 381K)
- The term aquaregia is a Latin phrase meaning ‘King’s Water’.
- The name reflects the ability of aquaregia to dissolve the noble metals such as gold, platinum and palladium.
Uses of Aquaregia
- It is used chiefly to dissolve metals such as gold and platinum.
- It is used for cleaning and refining gold.
Bases
- According to Arrhenius theory, bases are substances that ionise in water to form hydroxyl ions (OH–).
- There are some metal oxides which give salt and water on reaction with acids. These are also called bases.
- Bases that are soluble in water are called alkalis.
- A base reacts with an acid to give salt and water only.
![]()
- For example, zinc oxide (ZnO) reacts with HCl to give the salt zinc chloride and water.
![]()
- Similarly, sodium hydroxide ionises in water to give hydroxyl ions and thus get dissolved in water.
- So it is an alkali.
![]()
- Bases contain one or more replaceable oxide or hydroxyl ions in solution.
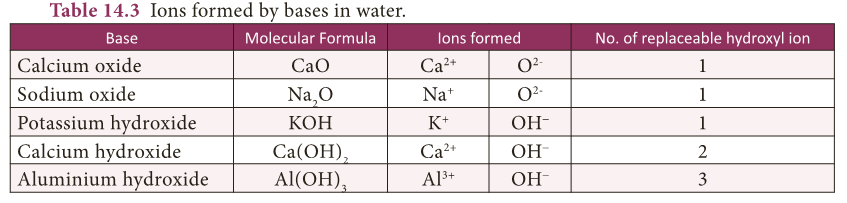
- Table 14.3 enlists various bases and ions formed by them in water.
Classification of Bases
(a) Based on their Acidity
- Monoacidic Base: It is a base that ionises in water to give one hydroxide ion per molecule. Example: NaOH, KOH
- Diacidic Base: It is a base that ionises in water to give two hydroxide ions per molecule. Example: Ca(OH)2. Mg(OH)2
- Triacidic Base: It is a base that ionises in water to give three hydroxide ions per molecule. Example: Al(OH)3, Fe(OH)3
(b) Based on concentration
- Concentrated Alkali: It is an alkali having a relatively high percentage of alkali in its aqueous solution.
- Dilute Alkali: It is an alkali having a relatively low percentage of alkali in its aqueous solution.
(c) Based on Ionisation
- Strong Bases: These are bases which ionise completely in aqueous solution. Example: NaOH, KOH
- Weak Bases: These are bases that ionise partially in aqueous solution. Example: NH4OH, Ca(OH)2
Properties of Bases
- They have bitter taste.
- Their aqueous solutions have soapy touch.
- They turn red litmus blue.
- Their aqueous solutions conduct electricity.
- Bases react with metals to form salt with the liberation of hydrogen gas.
![]()
- Bases react with non-metallic oxides to produce salt and water. Since this is similar to the reaction between a base and an acid, we can conclude that non- metallic oxides are acidic in nature.
![]()
- Bases react with acids to form salt and water. The above reaction between a base and an acid is known as Neutralisation reaction.
![]()
- On heating with ammonium salts, bases give ammonia gas.
![]()
Activity 2
- Take solutions of hydrochloric acid or sulphuric acid. Fix two nails on a cork and place the cork in a 100 ml beaker.
- Connect the nails to the two terminals of a 6V battery through a bulb and a switch as shown in Figure.
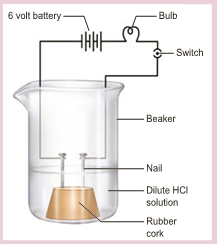
- Now pour some dilute HCl in the beaker and switch on the current.
- Repeat the activity with dilute sulphuric acid, glucose and alcohol solutions.
- What do you observe now?
- Does the bulb glow in all cases?
- In the above activity you can observe that the bulb will start glowing only in the case of acids.
- But, you will observe that glucose and alcohol solution do not conduct electricity.
- Glowing of the bulb indicates that there is a flow of electric current through the solution.
- The electric current is carried through the solution by ions.
- Repeat the same activity using alkalis such as sodium hydroxide and calcium hydroxide.
- Try Yourself: Construct a Lemon cell as shown in picture. Copper wire Copper coated coin
Uses of Bases
- Sodium hydroxide is used in the manufacture of soap.
- Calcium hydroxide is used in white washing of building.
- Magnesium hydroxide is used as a medicine for stomach disorder.
- Ammonium hydroxide is used to remove grease stains from cloths.
Tests for Acids and Bases
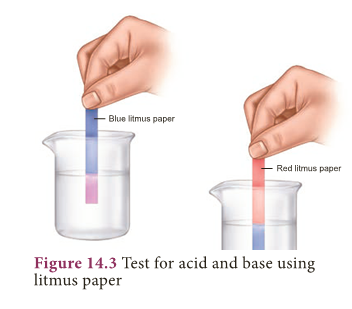
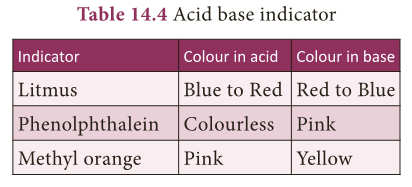
- Test with a litmus paper: An acid turns blue litmus paper into red. A base turns red litmus paper into blue.
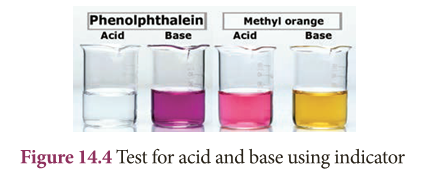
- Test with an indicator Phenolphthalein: In acid medium, phenolphthalein is colourless. In basic medium, phenolphthalein is pink in colour.
- Test with an indicator Methyl orange: In acid medium, methyl orange is pink in colour. In basic medium, methyl orange is yellow in colour.
Activity 3
- Collect the following samples from the science laboratory – Hydrochloric acid, Sulphuric acid and Nitric acid, Sodium hydroxide, Potassium hydroxide.
- Take 2 ml of each solution in a test tube and test with a litmus paper and indicators phenolphthalein and Methyl orange.
- Tabulate your observations.
Strenght of Acidic or Basic solutions
pH Scale
- A scale for measuring hydrogen ion concentration in a solution is called pH scale.
- The ‘p’ in pH stands for ‘potenz’ in German meaning power.
- pH scale is a set of numbers from 0 to 14 which is used to indicate whether a solution is acidic, basic or neutral.
- Acids have pH less than 7
- Bases have pH greater than 7
- A neutral solution has pH equal to 7
Salts
- When you say salt, you may think of the common salt.
- Sea water contains many salts dissolved in it.
- Sodium chloride is separated from these salts.
- There are many other salts used in other fields.
- Salts are the products of the reaction between acids and bases.
- Salts produce positive ions and negative ions when dissolved in water.
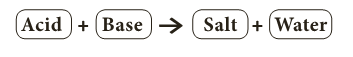
Types of Salts
- Normal Salts: A normal salt is obtained by complete neutralization of an acid by a base.
![]()
- Acid Salts: It is derived from the partial replacement of hydrogen ions of an acid by a metal.
![]()
- When a calculated amount of a base is added to a polybasic acid, acid salt is obtained.
- Basic Salts: Basic salts are formed by the partial replacement of hydroxide ions of a diacidic or triacidic base with an acid radical.
![]()
- Double Salts: Double salts are formed by the combination of the saturated solution of two simple salts in equimolar ratio followed by crystallization. For example, potash alum is a mixture of potassium sulphate and aluminium sulphate.
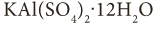
Properties of Salts
- Salts are mostly solids which melt as well as boil at high temperature.
- Most of the salts are soluble in water. For example, chloride salts of potassium and sodium are soluble in water.
- But, silver chloride is insoluble in water
- They are odourless, mostly white, cubic crystals or crystalline powder with salty taste.
- Salt is hygroscopic in nature.
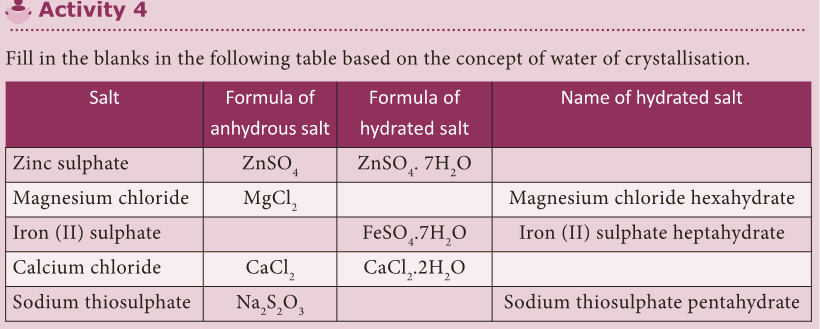
Water of Crystallisation
- Many salts are found as crystals with water molecules. These water molecules are known as water of crystallisation.
- Salts that contain water of crystallisation are called hydrated salts.
- The number of molecules of water hydrated to a salt is indicated after a dot in its chemical formula.
- For example, copper sulphate crystal have five molecules of water for each molecule of copper sulphate.
- It is written as CuSO4.5H2O and named as copper sulphate pentahydrate.
- This water of crystallisation makes the copper sulphate blue.
- When it is heated, it loses its water molecules and becomes white.
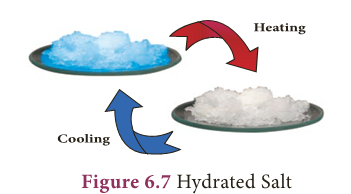
- Salts that do not contain water of crystallisation are called anhydrous salt. They are generally found as powders.
- Fill in the blanks in the following table based on the concept of water of crystallisation.
Identification of Salts
(i) Physical examination of the salt.
- The physical examination of the unknown salt involves the study of colour, smell and density. This test is not much reliable.
(ii) Dry heating Test.
- This test is performed by heating a small amount of salt in a dry test tube.
- After all the water get evaporated, the dissolved salts are sedimented in the container.
(iii) Flame Test.
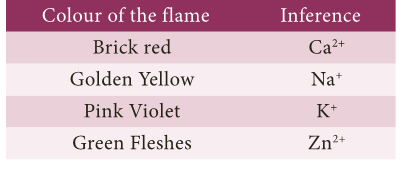
- Certain salts on reacting with concentrated hydrochloric acid (HCl) form their chlorides.
- The paste of the mixture with con.
- HCl is introduced into the flame with the help of platinum wire.
- When HCl is added with a carbonate salt, it gives off CO2 gas with brisk effervescence.
Uses of Salts
Common Salt (Sodium Chloride – NaCl)
- It is used in our daily food and used as a preservative.
Washing Soda (Sodium Carbonate-Na2CO3)
- It is used in softening hard water.
- It is used in glass, soap and paper industries.
Baking Soda (Sodium bicarbonate -NaHCO3)
- It is used in making of baking powder which is a mixture of baking soda and tartaric acid.
- It is used in soda-acid fire extinguishers.
- Baking powder is used to make cakes and bread, soft and spongy.
- It neutralizes excess acid in the stomach and provides relief.
Bleaching powder (Calcium Oxychloride – CaOCl2)
- It is used as disinfectant.
- It is used in textile industry for bleaching cotton and linen.
Plaster of Paris (Calcium Sulphate Hemihydrate – CaSO4 .½ H2O)
- It is used for plastering bones.
- It is used for making casts for statues.
Activity 5
- Boil about 100 ml of ground water in a vessel to dryness.
- After all the water get evaporated observe the inner wall of the vessel.
- Can you observe any deposits?
- This is the deposit of dissolved salts present in water.
MORE TO KNOW:
FACTS:
- All acids essentially contain one or more hydrogens.
- But all the hydrogen containing substances are not acids.
- For example, methane (CH4) and ammonia (NH3) also contain hydrogen.
- But they do not produce H+ ions in aqueous solution.
- Few metals do not react with acid and liberate hydrogen gas. For example: Ag, Cu.
- All alkalis are bases but not all bases are alkalis. For example: NaOH and KOH are alkalis whereas Al(OH)3 and Zn(OH)2 are bases.
- The term acidity is used for base, which means the number of replaceable hydroxyl groups present in one molecule of a base.
- Few metals do not react with sodium hydroxide. Example: Cu, Ag, Cr
Monobasic
- For acids, we use the term basicity that refers to the number of replaceable hydrogen atoms present in one molecule of an acid.
- For example, acetic acid (CH3COOH) has four hydrogen atoms but only one can be replaced.
- Hence it is monobasic.
Ionisation
- Ionisation is the condition of being dissociated into ions by heat or radiation or chemical reactions or electrical discharge.
Role of water in acid solution
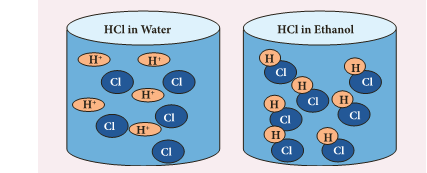
- Acids show their properties only when dissolved in water.
- In water, they ionise to form H+ ions which determine the properties of acids.
- They do not ionise in organic solvents.
- For example, when HCl is dissolved in water it produces H+ ions and Cl_ ions whereas in organic solvents like ethanol they do not ionise and remain as molecule.
EXTRA POINTS:
- Acids: Substance which furnishes H+ ions H3O+ ions when dissolved in water.
- Bases: Substance which furnishes OH- ions when dissolved in water.
- Salts: Product of reaction between acids and bases.
- Indicators: Chemical substances used to find out whether the given solution is acid or base.
- pH Scale: Scale used to find out Hydrogen ion concentration in a solution.
- pH Paper: Paper used to find out whether the given solution is acidie or basic or neutral in nature.
- Aquaregia: Mixture of hydrochloric acid and nitric acid prepared optimally in a molar ratio of 3 : 1.
- Hygroscopic substance: Substance which absorbs water from the surroundings.