8th Std Science Lesson Wise Questions in English – Part 3
8th Science Lesson 19 Questions in English
19] Acids And Bases
1. The food substances like Tamarind, curd and lemon has a ____ taste and said to be ____ in nature.
a) Sour, Acidic
b) Sweet, Acidic
c) Bitter, Base
d) Spicy, Base
Explanation
In our daily life we come across different food substances. Some substances like fruits, tamarind, graphs, curd and lemon are sour. They are said to be acidic.
2. Which of these substances are not classified as base?
a) Green tea
b) Lemon
c) Grapes
d) Soaps
Explanation
Some substances like sodium bicarbonate and green tea are bitter in taste. They are said to be basic.
3. Choose the correct statements.
i) Acid and bases are one of the important chemicals used in every field of science and in daily life.
ii) Acid and base does not react with each other.
iii) Aspirin and dietary fats are classified as acids.
iv) All the biological molecules are classified as bases.
v) The chemical compounds in DNA are base.
a) i, iii, iv, v only
b) ii, iii, v only
c) ii, iv, v only
d) i, iii, v only
Explanation
Acids and bases are one of the important chemical compounds which play a significant role in every field of science. Acids and bases are everywhere right from the soap used for shower to the vinegar present in the kitchen. Acids and bases react with each other and also with water. As a result they are important biologically, industrially and environmentally. For example, among the medicines we use, aspirin is acidic and antacids are basic. Similarly, many biological molecules are also either acids or bases. Dietary fats are acids and the chemical compounds in DNA are bases.
4. Assertion (A): The chemical compounds with sour taste are called acids generally.
Reasoning (R): Acid is the term derived from the Latin word Acidus which means Sour.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
The term acid is derived from the Latin word ‘acidus’ which means sour. Thus, the chemical compounds which have sour taste are called acids generally.
5. Which of these ions are released from the acids when dissolved in water?
a) CH
b) H+
c) H2
d) CH-
Explanation
All acids contain one or more replaceable hydrogen atoms in their molecules and when dissolved in water they release H+ ions.
6. Which of the following does not release hydrogen ions when dissolved in water?
a) Sodium hydroxide
b) Sulphuric Acid
c) Nitric Acid
d) Hydrochloric Acid
Explanation
Hydrochloric acid (HCl), Sulphuric acid (H2SO4) and Nitric acid (HNO3) release hydrogen ions (H+) when dissolved in water.
7. According to whose theory the substance which furnishes H+ or H3O+ ions in aqueous solution is acid?
a) Gilbert Lewis
b) Antoine Lavoisier
c) Svante Arrhenius
d) Dmitri Mendeleev
Explanation
Swedish chemist Svante Arrhenius proposed a theory on acids. According to him, an acid is a substance which furnishes H+ ions or H3O+ ions in aqueous solution.
8. On what basis acids are classified into organic and inorganic acids?
a) Chemical compounds
b) Source
c) Physical properties
d) Dissolving capacity
Explanation
Acids are defined as the chemical substances which release hydrogen ions when dissolved in water. Acids can be classified into organic acids and inorganic acids depending on the sources.
9. Which of the following is not an Organic acid?
a) Citric Acid
b) Hydrochloric Acid
c) Tartaric Acid
d) Oxalic Acid
Explanation
Some acids occur naturally in fruits and vegetables. These are called organic acids. Examples: Citric acid, tartaric acid etc.
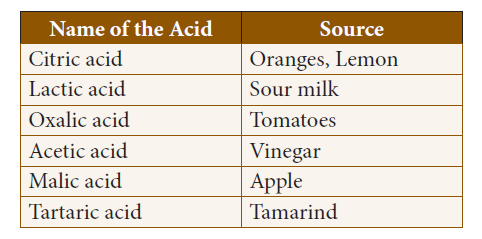
10. Identify the Incorrect Match.
A. Tomato i) Malic acid
B. Milk ii) Oxalic acid
C. Apple iii) Citric acid
D. Orange iv) Lactic acid
a) iv, i, ii, iii
b) iii, i, iv, ii
c) ii, iv, i, iii
d) ii, iii, iv, i
Explanation
Organic acids and their sources
11. Choose the correct statements.
i) Acids produced artificially in industries are called as Mineral acids or Inorganic acids.
ii) Organic and Inorganic acids are the only classification of acids.
iii) Hydrochloric acid, Sulphuric acid and Nitric acids are Organic acids.
a) i only
b) ii only
c) iii only
d) All the above
Explanation
On the other hand, man produces acids artificially in industries. These acids are called mineral acids or inorganic acids. Examples: Hydrochloric acid (HCl), Sulphuric acid (H2SO4), Nitric acid (HNO3) etc., There are many more classifications of acids.
12. Which of the following property is not suitable for an acid?
a) Acids are sour in taste.
b) Acids are soluble in water.
c) Acids are colorless.
d) Acids are non-corrosive in nature.
Explanation
Physical properties of Acids: Acids are sour in taste, they are corrosive in nature. Acids are colorless. They are soluble in water.
13. Choose the correct statements.
i) The solutions of acids conduct electricity.
ii) All acids will spoil the substances like clothes, paper and human skin.
a) i only
b) ii only
c) Both i and ii
d) Neither i nor ii
Explanation
Strong acids can spoil substances like human skin, clothes and paper. Solutions of acids conduct electricity.
14. Which of these acids exist in solid state?
a) Benzoic acid
b) Citric acid
c) Sulphuric acid
d) Nitric acid
Explanation
Generally acids exist in liquid state but few acids exist in solid state too. E.g. Benzoic acid
15. Which of these is changed to pink color if treated with acids?
a) Blue litmus
b) Phenolphthalein
c) Methyl orange
d) Red litmus
Explanation
Acids change the color of the indicators. Blue litmus paper turns red and methyl orange turns pink when treated with acids.
16. Which of this gas is released in the acid-metal reaction?
a) Oxygen
b) Hydrogen
c) Nitrogen
d) Carbon dioxide
Explanation
Metals like zinc, magnesium, aluminum, iron etc., react with acids like hydrochloric acid, Sulphuric acid to form metal salts and release hydrogen gas.
Metal + Dilute acids → Metal salt + Hydrogen
17. Choose the Incorrect statements.
i) All the cooking vessels are coated with tin metal.
ii) Metal coating prevents the chemical reaction of the organic acids of food materials with the vessel materials.
iii) Tin isolates the vessels from the acid reaction and prevents food poisoning.
a) i only
b) ii only
c) iii only
d) None of the above
Explanation
Copper or brass cooking vessels are coated with tin metal (eyam). If it is not coated the organic acids present in the food materials will react with copper and make the food poisonous. The tin isolates the vessel from the action of acids and prevents food poisoning.
18. Which of these results in the reaction of limestone and Sulphuric acid?
a) Calcium sulphate
b) Water
c) Carbon dioxide
d) All the above
Explanation
When carbonates and bicarbonates come into contact with dilute acids carbon dioxide is given out along with water. For example limestone (calcium carbonate) reacts with dilute Sulphuric acid to form calcium sulphate, carbon dioxide and water.
Calcium carbonate + dil Sulphuric acid → Calcium sulphate + Carbon dioxide + Water
CaCO3 + H2SO4 → CaSO4 + CO2 + H2O
19. Which of these reacts with acids and produce metal salts and water?
a) Metal oxides
b) Metals
c) Minerals
d) Metal sulphates
Explanation
Oxides of various metals react with dilute acids to form their metallic salts and water.
Metal oxides + dilute Acid → Metal salts + Water
20. Match
A. Fatty acids i) Food digestion in stomach
B. Acetic acid ii) Pickles
C. Hydrochloric acid iii) Preserve food materials
D. Benzoic acid iv) Soaps
a) ii, iii, iv, i
b) iv, iii, i, ii
c) i, iv, ii, iii
d) ii, i, iii, iv
Explanation
Uses of Acids
- Hydrochloric acid present in our stomach helps in the digestion of foodstuff.
- Vinegar (acetic acid) is used to preserve food materials.
- Benzoic acid is also used to preserve food materials like pickles.
- Sodium or potassium salts of higher fatty acids are used to make washing and bathing soaps.
21. Which of the following acid is called as the king of chemicals?
a) Sulphuric acid
b) Hydrochloric acid
c) Nitric acid
d) Acetic acid
Explanation
Sulphuric acid is called the king of chemicals. It is used in various industries to make detergents, paints, fertilizers and many more chemicals.
22. What is the usage of the Sulphuric acid?
a) Pain killer
b) Food preserving agent
c) Dehydrating agent
d) All the above
Explanation
Sulphuric acid is an effective dehydrating agent. It is used in various industries to make detergents, paints, fertilizers and many more chemicals.
23. Which of the following is not a laboratory reagent?
a) Hydrochloric acid
b) Nitric acid
c) Citric acid
d) Sulphuric acid
Explanation
Hydrochloric acid, Nitric acid and Sulphuric acid are important laboratory reagents.
24. Assertion (A): Cells of all the living organisms contains the fundamental nuclear material called as nucleic acids.
Reasoning (R): Animals have deoxy ribonucleic acid and plants contain ribonucleic acids.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
Cells of all living organisms contain the fundamental nuclear material called nucleic acids. Animals have deoxy ribonucleic acid (DNA) whereas plants contain ribonucleic acid (RNA).
25. Which of the following is used to maintain pickles in good condition for a long time?
a) Hydrochloric acid
b) Tartaric acid
c) Acetic acid
d) Sulphuric acid
Explanation
Pickles remain in good condition for long time because they contain vinegar (acetic acid) or benzoic acid.
26. Assertion (A): Most of the soaps, detergents, toothpastes are the examples of base.
Reasoning (R): The slippery texture of the soaps is due to the presence of base.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
Soaps are slippery due to the presence of ‘base’. Bases are chemical substances that are corrosive and bitter in taste. A lot of bleaches, soaps, detergents, kinds of toothpaste, etc., are bases. In contrast to acids which release hydrogen ions in water, bases release hydroxide ions in water.
27. Choose the correct statements.
i) Base is a chemical substance that releases hydroxide ions when dissolved in water.
ii) All the soluble bases are called as alkalis.
a) i only
b) ii only
c) Both i and ii
d) Neither i nor ii
Explanation
The chemical substances that release hydroxide ions when dissolved in water are called as bases. Examples: Sodium hydroxide (NaOH) and Potassium hydroxide (KOH).
Sodium hydroxide → Sodium ion + Hydroxide ion
NaOH → Na+ + OH–
28. Which of the following is not an alkali?
a) Potassium hydroxide
b) Aluminium hydroxide
c) Ammonium hydroxide
d) Sodium hydroxide
Explanation
Water soluble bases are called Alkalis. Bases like sodium hydroxide, potassium hydroxide, calcium hydroxide and ammonium hydroxide are highly soluble in water and hence they are called alkalis.
29. Which of the following base does not release hydroxide ions if dissolved in water?
a) Sodium carbonate
b) Sodium bicarbonate
c) Calcium carbonate
d) All the above
Explanation
Certain chemical substances which do not release hydroxide ions when dissolved in water also behave as bases. Examples: Sodium carbonate, Sodium bicarbonate, Calcium carbonate etc.
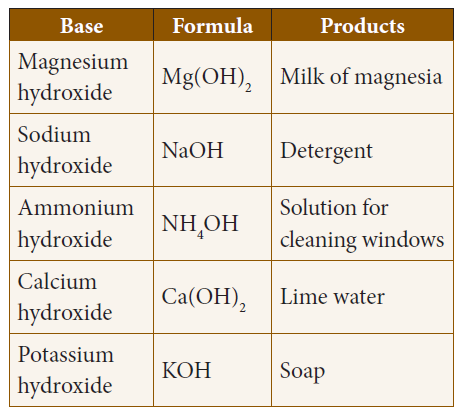
30. Match.
A. Magnesia milk i) Calcium hydroxide
B. Detergent ii) Magnesium hydroxide
C. Lime water iii) Ammonium hydroxide
D. Window cleaning solution iv) Sodium hydroxide
a) iii, ii, iv, i
b) iv, i, iii, ii
c) ii, iv, i, iii
d) ii, iii, i, iv
Explanation
Common bases in some products
31. Identify the incorrect match.
A. Caustic potash i) Ammonium hydroxide
B. Sodium bicarbonate ii) Baking soda
C. Sodium hydroxide iii) Caustic soda
D. Sodium carbonate iv) Washing soda
a) i only
b) ii only
c) iii only
d) iv only
Explanation
Sodium carbonate (Na2CO3) is commercially called washing soda. Similarly sodium bicarbonate (NaHCO3) is commercially called baking soda. Caustic soda is sodium hydroxide (NaOH) and caustic potash is potassium hydroxide (KOH).
32. Which of the following base exist in liquid state?
a) Potassium hydroxide
b) Sodium hydroxide
c) Calcium hydroxide
d) Aluminium hydroxide
Explanation
Properties of Bases: Bases generally exist in solid state but some bases exist in liquid state also. E.g. Ammonium hydroxide, calcium hydroxide
33. Which of the following is physical property of a base?
i) Base gives soapy touch in all medium.
ii) Bases are corrosive in nature.
iii) Bases are colorless.
iv) Bases are bitter in taste.
a) i, ii only
b) ii, iii, iv only
c) iii only
d) ii, iv only
Explanation
Physical properties of Base: Bases give soapy touch only in aqueous media not in dry nature. Bases are bitter in taste. Bases are corrosive in nature. When come in contact with the skin frequently they form painful blisters. Bases are generally colorless. Bases also conduct electricity in aqueous solution.
34. Match the color indicators of the base.
A. Methyl orange i) Blue
B. Red litmus ii) Pink
C. Phenolphthalein iii) Yellow
a) iii, i, ii
b) i, iii, ii
c) ii, i, iii
d) ii, iii, i
Explanation
Bases also change the color of the indicators. Red litmus paper turns blue when treated with bases. Similarly, they turn methyl orange yellow and phenolphthalein pink.
35. Assertion (A): In general all the existing metals react with a base.
Reasoning (R): A metallic oxide does not react with base.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
Reaction with metals: Generally metals do not react with bases. All bases react with non- metallic oxides to form salt and water.
36. Which of the following metal react with base and release hydrogen?
a) Tin
b) Zinc
c) Lead
d) Copper
Explanation
Reaction with metals: Generally metals do not react with bases. Metals like Aluminium and Zinc react with bases like sodium hydroxide forming aluminates and release hydrogen.
Aluminum + Sodium hydroxide + Water → Sodium aluminate + Hydrogen
2Al + 2NaOH + 2H2O → 2NaAlO2+ 3H2
37. Which of these reacts with base and results with salt and water?
a) Metals
b) Hydrogen
c) Metal oxides
d) Carbon
Explanation
Reaction with metal oxides: All bases react with non-metallic oxides to form salt and water. For example sodium hydroxide reacts with carbon dioxide to form sodium carbonate.
Sodium hydroxide + Carbon dioxide → Sodium carbonate + Water
2NaOH + CO2 → Na2CO3 + H2O
38. Which of these does not results in from the reaction of base with ammonium salts?
a) Carbon dioxide
b) Ammonia
c) Metal salts
d) Water
Explanation
Bases react with ammonium salts to form metal salts, ammonia gas and water.
Sodium hydroxide + Ammonium chloride → Sodium chloride + Ammonia + Water
NH4Cl + NaOH → NaCl + NH3 + H2O
39. Which is not a common property of an acid and base?
a) In aqueous solution they conduct electricity.
b) Corrosive in nature.
c) Tastes sour.
d) Undergo ionization in aqueous solution.
Explanation
Though acids and bases have some unique properties there are certain similarities between them. Some of them are given below.
- They are corrosive in nature.
- They undergo ionization in aqueous solution.
- They conduct electricity in aqueous solution.
- They undergo neutralization reaction
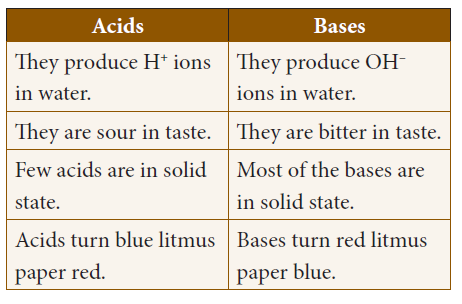
40. Choose the correct statements.
i) Acids are sour in taste and produce H+ ions in water.
ii) Base turns red litmus paper to green color.
iii) Most of the base is in liquid state.
iv) Very few acids are in solid state.
a) i, iv only
b) ii, iii, iv only
c) ii, iv only
d) i, iii, iv only
Explanation
Difference between acids and bases
41. Which of the following statements is not true?
a) Potassium hydroxide is used for making bathing soaps.
b) Calcium hydroxide is used for white washing.
c) Aluminium hydroxide is used in paper industries.
d) Sodium hydroxide is used to make washing soaps.
Explanation
Uses of Bases: Potassium hydroxide is used to make bathing soaps. Sodium hydroxide is used to make washing soaps. Sodium hydroxide is also used in paper industries, textile industries and in the preparation of medicines. Calcium hydroxide is used for white washing.
42. What is used in antacids?
a) Aluminium hydroxide
b) Ammonium hydroxide
c) Calcium hydroxide
d) Sodium hydroxide
Explanation
Aluminum hydroxide and magnesium hydroxides are used in antacids to cure acidity problems.
43. Which of these are manufactured by using the ammonium hydroxide?
a) Nylon
b) Fertilizers
c) Rubber and plastics
d) All the above
Explanation
Ammonium hydroxide is used to manufacture fertilizers, nylon, plastics and rubber.
44. Which of these results in from the neutralization reaction of acid and base?
a) Salt
b) Steam
c) Water
d) Both a and c
Explanation
When neutrality is achieved between two different chemical substances with different chemical properties through a reaction then it is called neutralization in chemistry. Thus neutralization is a chemical reaction in which an acid and a base react with each other to form salt and water. Neutralization reaction between an acid and a base can be written as: Acid + Base → Salt + Water
45. Which of this salt is produced by the neutralization reaction of Nitric acid and Sodium hydroxide?
a) Sodium nitrate
b) Sodium acetate
c) Sodium sulphate
d) Sodium chloride
46. Which of this acid reacts with sodium hydroxide to produce Sodium sulphate?
a) Citric Acid
b) Sulphuric Acid
c) Hydrochloric Acid
d) Oxalic Acid
Explanation
Salts produced by neutralization

47. Assertion (A): An Ant bite injects formic acid causing burning sensation and pain.
Reasoning (R): Calcium hydroxide is applied to neutralize the effects of formic acid.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
Ant bite: Whenever bees or red ants bite they inject an acid called formic acid. These acids cause burning sensation and pain. To suppress the pain a suitable base in the form of calcium hydroxide (readily available at home) is applied so as to neutralize the formic acid.
48. Which of the following neutralizes the alkalinity of wasp bite?
a) Lime
b) Turmeric
c) Vinegar
d) Beetroot
Explanation
When we are bitten by wasp, we feel the burning sensation and pain. It is due to an alkaline substance injected by the insect. To neutralize the alkalinity we use vinegar which is an acid.
49. The Tooth powder and pastes contains _____ to neutralize the ____ effects of tooth decay.
a) Weak Base, Acid
b) Strong Acid, Base
c) Strong Base, Acid
d) Weak Acid, Base
Explanation
Generally it is advised by the doctors that we should brush our teeth twice a day. This is because the bacteria present in our mouth decompose the food particles stuck in the gaps between our teeth thereby causing acid formation which leads to tooth decay. To prevent this we have to neutralize the acid. When we brush with tooth powder or tooth paste containing weak bases the acid gets neutralized. So our teeth will be strong and healthy.
50. Choose the correct statements.
i) Hydrochloric acid present in human stomach helps to digest the food materials using the enzymes.
ii) Excessive hydrochloric acid in stomach results in burning sensation in food pipes and ulcer.
iii) Aluminium and magnesium hydroxides are weak bases used to reduce the acidic effects of stomach.
a) i only
b) ii only
c) iii only
d) All the above
Explanation
As we know, hydrochloric acid present in our stomach helps the digestion of food material along with the enzymes secreted by liver, gallbladder and pancreases. Sometimes due to excessive production of hydrochloric acid in our stomach we feel burning sensation in food pipe and in chest area. If this happens again and again ulcer will be formed in stomach and food pipe, which further aggravates the conditions. In order to neutralize, antacids which are nothing but weak bases like aluminum and magnesium hydroxides are used. As a result the acidity is removed.
51. Which of these does not reduce the acidic effects of soil?
a) Limestone
b) Nitrogen
c) CaO
d) Burnt wood ash
Explanation
Acidic soil is not suitable for plant growth. So farmers add lime fertilizers such as powdered lime (CaO), limestone (CaCO3) or ashes of burnt wood to the soil to neutralize the acidity.
52. Which of these are added to neutralize the effects of acidic effluents from the industries?
a) Lime
b) Limestone
c) Powdered Lime
d) All the above
Explanation
Effluents from the industries contain acids such as Sulphuric acid. It is treated by adding lime to neutralize it before it is discharged into rivers and streams. Power stations treat this acidic gas using powdered lime (CaO) or limestone (CaCO3) to neutralize it so that air pollutant can be prevented.
53. Which of this gas is released while burning the fossil fuels?
a) Carbon dioxide
b) Sulphuric dioxide
c) Helium
d) Nitrogen
Explanation
In power stations fossil fuels such as coal are burnt to produce electricity. Burning fossil fuels will liberate sulphur dioxide gas as an acidic pollutant in the air.
54. Assertion (A): Acid-base indicator is a chemical substance that shows the acidic or basic nature of a solution.
Reasoning (R): Indicators may be natural or synthetic based on the source.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
An indicator or acid–base indicator is a chemical substance which indicates the acidic or basic nature of a solution by suitable color change. These may be natural or synthetic.
56. Which of the following is not a natural indicator?
a) Turmeric
b) Red cabbage
c) Methyl Orange
d) China rose petals
Explanation
Natural indicators are chemical substances which are obtained from the natural resources. Litmus, turmeric juice, China rose petals, red cabbage, grape juice and beetroot juice are the indicators obtained from natural resources.
57. Which of these indicator changes to red color in basic solution?
a) Cabbage
b) Turmeric
c) Beetroot juice
d) Grape juice
Explanation
Turmeric indicator: By adding small amount of water to turmeric powder a paste is prepared. This is applied on a blotting paper or filter paper and dried. These strips are used as indicators to find the nature of the solution. In acidic solution turmeric indicator paper has no change in color. That means it remains yellow. In basic solution the color changes from yellow to red.
58. Which of these color results when subjecting the hibiscus flower solution to a basic solution?
a) Red
b) Yellow
c) Green
d) Pink
Explanation
Some hibiscus flowers soaked in warm water for about 5 to 10 minutes forms a solution. This solution can be used as indicator. In acidic solution, the color will be changed to deep pink or deep red. In basic solution the color will be changed into a green.
59. Choose the correct statements.
i) Synthetic indicators are prepared from artificial substances.
ii) Phenolphthalein and methyl orange are the examples of synthetic indicators.
a) i only
b) ii only
c) Both i and ii
d) Neither i nor ii
Explanation
An indicator prepared from artificial substances is known as synthetic indicators. Phenolphthalein and methyl orange are the examples for synthetic indicators.
60. Choose the correct statements.
i) Phenolphthalein is a colorless compound.
ii) Phenolphthalein is mixed with hot water and used as an indicator.
a) i only
b) ii only
c) Both i and ii
d) Neither i nor ii
Explanation
Phenolphthalein is a colorless compound. Its alcoholic solution is used as an indicator. It is colorless in acidic solution but turns pink in basic solution.
61. Choose the Incorrect statements.
i) Solid Methyl Orange is directly used as an indicator.
ii) Methyl orange turns to red color in acidic solution.
iii) A basic solution changes the color of methyl orange to yellow color.
a) i only
b) ii only
c) iii only
d) None of the above
Explanation
Solid methyl orange is dissolved in hot water and its filtrate is used as an indicator. It turns red in acidic solution and yellow in basic solution.
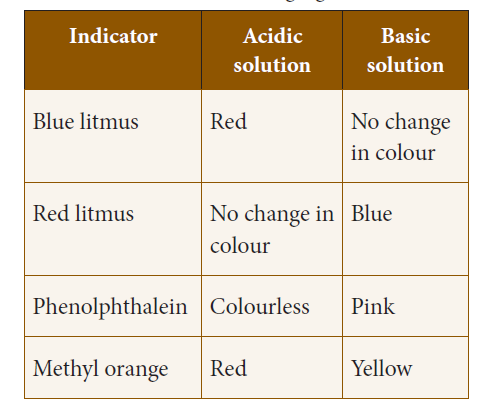
62. Match the Indicators color change in Basic solution.
A. Phenolphthalein i) No change
B. Blue Litmus ii) Blue
C. Methyl Orange iii) Pink
D. Red Litmus iv) Yellow
a) i, iv, ii, iii
b) iii, i, iv, ii
c) ii, iv, iii, i
d) iv, i, ii, iii
63. Which of these does not change in color in an acidic solution?
a) Blue Litmus
b) Phenolphthalein
c) Red Litmus
d) Methyl Orange
Explanation
Color Changing Indicators