8th Std Science Lesson Wise Questions in English – Part 2
8th Science Lesson 9 Questions in English
9] Heat
1. Which among the following is not the important changes that we can see in our daily life due to heat energy as it supplied to substance?
- Expansion
- Change in atomic form
- Change in state
- Increase in temperature
Explanation
When heat energy is supplied to any substance, it brings about many changes. There are three important changes that we can see in our daily life. They are: 1. Expansion 2. Increase in temperature and 3. Change in state.
2. Which among the following statement is correct
- All the substances in our surrounding are made up of atoms and molecules. These atoms and molecules are always at static position. These static substances have an energy known as heat energy. This energy flows from cold substances to hot substances or from cold region to hot region of a substance.
- When heat energy is supplied to any substance it increases the energy of the atoms and molecules in it and so they start to vibrate. These atoms and molecules which vibrate make other atoms and molecules to vibrate. Thus, heat energy is transferred from one part of the substance to other part. We can see this heat energy transfer in our daily life also. Heat energy brings about lot of changes.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
All the substances in our surrounding are made up of atoms and molecules. These atoms and molecules are always at vibratory motion. Due to this motion substances have an energy known as heat energy. This energy flows from hot substances to cold substances or from hot region to cold region of a substance.
3. When the water is heated, water molecules receive heat energy. This heat energy supplied increases which of the molecules?
- Gravitational energy
- Potential energy
- Kinetic energy
- Frictional energy
Explanation
When the water is heated, water molecules receive heat energy. This heat energy supplied increases the kinetic energy of the molecules. When the molecules receive more energy, the temperature of the water increases. This shows that heat energy causes increase in temperature.
4. In ice cubes the force of attraction between the water molecules is ___
- More
- Less
- Nil
- None of the above
Explanation
In ice cubes the force of attraction between the water molecules is more. So, they are close together. When we heat them the force of attraction between the molecules decreases and the ice cubes become water.
5. Which among the following is incorrect regarding the Expansion effect of heat
- Take a metal ball and a metal ring of suitable diameter. Pass the metal ball through the ring. You can observe that the metal ball can easily go through it. Now heat the metal ball and then try to pass it through the ring. It will not pass through the ring. Keep the metal ball on the ring for some time. In few minutes, it will fall through the ring
- When the ball is heated the atoms in the ball gain heat energy. They start vibrating and force each other apart. As a result, an expansion takes place. That’s why the ball did not go through the ring.
- After some time, as the ball lost the heat energy to the surrounding it came back to its original size and it went through the ring. This shows that heat energy causes expansion and contraction in solids. This expansion takes place only in solid not in liquids and gases also. It is very rare in gases.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
After some time, as the ball lost the heat energy to the surrounding it came back to its original size and it went through the ring. This shows that heat energy causes expansion in solids. This expansion takes place in liquids and gases also. It is maximum in gases.
6. Which among the following statement is correct
- When we heat the water, the force of attraction decreases further. Hence they move away from one another and become vapour. Since water vapour escape to the surrounding, water level decreases further. From this we understand that heat energy causes change in the state of the substances.
- When heat energy is removed, changes take place in reverse direction. If heat energy is supplied to or taken out from a substance, it will undergo a change from one state of matter to another. There are six transformations may take place due to heat energy.
- Only 1
- Only 2
- Both 1 and 2
- None
7. The solid to gas transformation is known as ____
- Vaporisation
- Condensation
- Deposition
- Sublimation
Explanation
The solid to gaseous transformation is known as Sublimation.
8. The transformation of gas to sloid state is known as ____
- Vaporisation
- Condensation
- Deposition
- Freezing
Explanation
The transformation of gas to solid state is known as Deposition.
9. The transformation of solid to liquid state is called as _____
- Condensation
- Freezing
- Melting
- Vaporisation
Explanation
The transformation of solid to liquid state is called as Melting.
10. The Transformation of gas to liquid state is known as ____
- Vaporisation
- Condensation
- Deposition
- Melting
Explanation
The transformation of gas to liquid state is known as condensation.
11. Which among the following is not transformation take place due to heat energy
- Vaporisation
- Freezing
- Condensation
- Conservation
Explanation
The transformations may take place due to heat energy. 1. Solid to Liquid (Melting) 2. Liquid to Gas (Vaporisation) 3. Solid to Gas (Sublimation) 4. Gas to Liquid (Condensation) 5. Liquid to Solid (Freezing) 6. Gas to Solid (Deposition).
12. Heat does not transfer by which among the following way?
- Conduction
- Compression
- Convection
- Radiation
Explanation
If heat energy is supplied to any substance, it will be transferred from one part of the substance to another part. It takes place in different ways depending on the state of the substance. Three ways of heat transfer are: 1. Conduction 2. Convection and 3. Radiation.
13. Which among the following statement is correct
- Leave the spoon inside the hot water for some time. The other end of the spoon become hot. It is because heat in the hot water is transferred from one end to another end of the spoon.
- In solid substances such as silver spoon, atoms are loosely arranged. Hot water molecules which are vibrating transfer the heat energy to the atoms in the spoon and make them vibrate. Those atoms make other atoms to vibrate and thus heat is transferred to the centre of the spoon.
- In conduction heat transfer takes place between two ends of the same solid or through two solid substances that are at different temperatures but in contact with one another. Thus, we can define conduction as the process of heat transfer in solids from the region of higher temperature to the region of lower temperature without the actual movement of atoms or molecules.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
In solid substances such as silver spoon, atoms are arranged very closely. Hot water molecules which are vibrating transfer the heat energy to the atoms in the spoon and make them vibrate. Those atoms make other atoms to vibrate and thus heat is transferred to the other end of the spoon.
14. Which is the only matter on the Earth that can be found naturally in all three states – Solid, Liquid and Gas?
- Lava
- Water
- Nitrogen
- All the above
Explanation
Water is the only matter on the Earth that can be found naturally in all three states – Solid, Liquid and Gas.
15. Which among the following is the bad conductor?
- Rubber
- Cobalt
- Nickle
- Titanium
Explanation
All metals are good conductors of heat. The substances which do not conduct heat easily are called bad conductors or insulators. Wood, cork, cotton, wool, glass, rubber, etc are insulators.
16. Which among the following is incorrect regarding conduction
- We cook food in vessels made up of metals. When the vessel is heated, heat is transferred from the metal to the food.
- When we iron dresses, heat is transferred from the iron to the cloth
- Handles of cooking utensils are made up of plastic or wood because they are poor conductors of heat
- The temperature inside igloo (snow house) is cool because snow is a good conductor of heat.
Explanation
The temperature inside igloo (snow house) is warm because snow is a poor conductor of heat.
17. Which among the following statement is incorrect
- When water in the vessel is heated, water molecules at the to receive heat energy and move downward. Then the molecules at the bottom comes top and get heated. This kind of heat transfer is known as convection. This is how air in the atmosphere is also heated.
- Thus, the form of heat transfer from places of high temperature to places of low temperature by the actual movement of molecules is called convection. Convection takes place in liquids and gases.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
When water in the vessel is heated, water molecules at the bottom receive heat energy and move upward. Then the molecules at the top comes down and get heated. This kind of heat transfer is known as convection. This is how air in the atmosphere is also heated.
18. Which among the following statement is correct regarding convection
- Formation of land breeze and sea breeze is due to convection of air.
- Wind flows from one region to another region by convection
- In hot air balloons heat is transferred by convection and so the balloon raises.
- In refrigerators, cool air moves upward and replaces the cool air because of convection.
Explanation
In refrigerators, cool air moves downward and replaces the hot air because of convection.
19. Which is defined as the way of heat transfer from one place to another in the form of electromagnetic waves?
- Convection
- Condensation
- Radiation
- Scattering
Explanation
Radiation is defined as the way of heat transfer from one place to another in the form of electromagnetic waves.
20. Radiation does not pass through which among the medium?
- Liquid
- Gas
- Vacuum
- None of the above
Explanation
Radiation is the third form of heat transfer. By conduction, heat is transferred through solids, by convection heat is transferred through liquids and gases, but by radiation heat can be transferred through empty space even through vacuum. Heat energy from the Sun reaches the Earth by this form of heat transfer.
21. Which among the following statement is correct
- Heat energy from the Sun reaches the Earth by radiation.
- While standing near fire we feel the heat which is transferred as radiation.
- Black surfaces reflect heat radiation. So that the bottom of the cooking vessels is painted black
- All the above
Explanation
Black surfaces absorb heat radiation. So that the bottom of the cooking vessels is painted black. White colour reflects heat radiation. That’s why we are advised to wear white cloth during summer.
22. When a substance is heated to what temperature the radiation begins to become visible to the eye as a dull red glow, and it is sensed as warmth by the skin?
- 1000 C
- 2000C
- 3500C
- 5000C
Explanation
Heat transfer by radiation is visible to our eyes. When a substance is heated to 500°C the radiation begins to become visible to the eye as a dull red glow, and it is sensed as warmth by the skin. Further heating rapidly increases the amount of radiation, and its perceived colour becomes orange, yellow and finally white.
23. The amount of energy in food items is measured by the unit _____
- Calorie
- Kilo Calorie
- Kilo Joule
- Joule
Explanation
The amount of energy in food items is measured by the unit kilo calorie.
1 kilo calorie = 4200 J (Approximately).
24. The technique used to measure the amount of heat involved in a physical or a chemical process is known as ____
- Calorimetry
- Alkalimetry
- Fluorometry
- Chronometry
Explanation
There are physical changes due to heat energy like ice transform to liquid. Similarly, heat energy produces chemical changes also. To know more about the physical and chemical changes that take place in substances, we need to measure the amount of heat involved. The technique used to measure the amount of heat involved in a physical or a chemical process is known as calorimetry.
25. Which is a physical quantity which expresses whether an object is hot or cold?
- Velocity
- Pressure
- Temperature
- Density
Explanation
Temperature is a physical quantity which expresses whether an object is hot or cold.
26. Temperature is measured with the help of _____
- Seismograph
- Thermometer
- Manometer
- Hygrometer
Explanation
Temperature is measured with the help of thermometer.
27. Which among the following is not the scale to measure the temperature?
- Celsius scale
- Fahrenheit scale
- Kelvin scale
- Fujita scale
Explanation
There are three scales to measure the temperature. They are: • Celcius scale • Fahrenheit scale • Kelvin scale.
28. The unit of energy in SI system is ____
- Newton
- Pascal
- Joule
- Watt
Explanation
We know that heat is a form of energy. The unit of energy in SI system is joule. So, heat is also measured in joule. It is expressed by the symbol J.
29. Which among the following is not the factor that determine the amount of heat energy gained or lost by a substance?
- Mass of the substance
- Change in temperature of the substance
- Nature of the material of the substance
- None of the above
Explanation
In general, the amount of heat energy gained or lost by a substance is determined by three factors. They are: • Mass of the substance • Change in temperature of the substance • Nature of the material of the substance.
30. Which is defined as the amount of heat energy required by a substance to raise its temperature by 1°C or 1 K?
- Heat capacity
- Kilo joule
- One calorie
- None of the above
Explanation
Different substances require different amount of heat energy to reach a particular temperature. This nature is known as heat capacity of a substance. Heat capacity is defined as the amount of heat energy required by a substance to raise its temperature by 1°C or 1 K. It is denoted by the symbol C’.
31. Which among the following is the correct formula of ; Heat capacity = ?
- Raise in temperature (ΔT) / Amount of heat energy required (Q)
- Amount of heat energy required (Q) / Raise in temperature (ΔT)
- Amount of heat energy required (Q) × Raise in temperature (ΔT)
- Raise in temperature (ΔT) + Amount of heat energy required (Q)
Explanation
Heat capacity = Amount of heat energy required (Q) / Raise in temperature (ΔT).
32. In the SI system, heat capacity is measures in ____
- C
- K
- JK-1
- JK-2
Explanation
The unit of heat capacity is Cal / °C. In SI system, it is measured in JK-1.
33. The temperature of a metal ball is 30°C. When an energy of 3000 J is supplied, its temperature raises by 40°C. Calculate its heat capacity?
- 90000 KJ-1
- 100 KJ-1
- 300 KJ-1
- 500 KJ-1
Explanation
Heat capacity, C’ = Q / ΔT
Here, Q = 3000 J
ΔT = 40°C – 30°C = 10°C = 10 K
C’ = 3000 / 100 = 300 JK-1
The heat capacity of the metal ball is 300 JK-1.
34. Which is the amount of heat energy required to raise the temperature of 1 gram of water through 1°C?
- One Newton
- One calorie
- Specific heat capacity
- Heat capacity
Explanation
The most commonly used unit of heat is calorie. One calorie is the amount of heat energy required to raise the temperature of 1 gram of water through 1°C.
35. The energy required to raise the temperature of an iron ball by 1 K is 500 JK-1. Calculate the amount of energy required to raise its temperature by 20 K?
- 10000 J
- 250 J
- 1000 J
- 480 J
Explanation
Heat capacity, C’ = Q / ΔT
Q = C’ × ΔT
Here, C’ = 500 JK-1
ΔT = 20 K
Q = 500 × 20 = 10000 J.
The amount of heat energy required is 10000 J.
36. Which is defined as the amount of heat energy required to raise the temperature of 1 kilogram of a substance by 1°C or 1 K?
- Heat capacity
- One calorie
- Specific heat capacity
- Joule capacity
Explanation
When the heat capacity of a substance is expressed for unit mass, it is called specific heat capacity. Specific heat capacity of a substance is defined as the amount of heat energy required to raise the temperature of 1 kilogram of a substance by 1°C or 1 K. It is denoted by the symbol C.
37. Which among the following is the correct equation for Specific heat capacity =?
- Amount of heat energy required (Q) × Mass × Raise in temperature (ΔT)
- (Amount of heat energy required (Q) × Mass) / Raise in temperature (ΔT)
- Amount of heat energy required (Q) / (Mass × Raise in temperature (ΔT))
- (Amount of heat energy required (Q) + Mass) / Raise in temperature (ΔT)
Explanation
Specific heat capacity =
Therefore, C =
38. What is the SI unit of specific heat capacity?
- J Kg K-1
- J Kg-1 K
- J Kg-1 K-1
- J-1 Kg-1 K-1
Explanation
The SI unit of specific heat capacity is J Kg-1 K -1.
39. An energy of 84000 J is required to raise the temperature of 2 kg of water from 60°C to 70°C. Calculate the specific heat capacity of water?
- 4200 J Kg-1 K-1
- 42000 J Kg-1 K-1
- 8400 J Kg-1 K-1
- 80000 J Kg-1 K-1
Explanation
Specific heat capacity, C = Q / m × ΔT
Here, Q = 84000 J
m = 2 kg
ΔT = 70°C – 60°C = 10°C = 10 K
C = 84000 / 2 × 10 = 4200 J Kg-1 K-1
The Specific heat capacity of water is 4200 J Kg-1 K-1.
40. Which is the most commonly used scale to measure Temperature?
- Celsius scale
- Fahrenheit scale
- Kelvin scale
- All the above
Explanation
Kelvin scale is the most commonly used one.
41. The specific heat capacity of a metal is 160 JKg-1 K-1. Calculate the amount of heat energy required to raise the temperature of 500 gram of the metal from 125°C to 325°C?
- 8000 J
- 32000 J
- 12000 J
- 16000 J
Explanation
Specific heat capacity, C = Q / m × ΔT
Q = C × m × ΔT
Here, C = 160 J Kg K-1
m = 500 g = 0.5 kg
ΔT = 325°C – 125°C = 200°C = 200 K
Q = 160 × 0.5 × 200 = 16000 J.
The amount of heat energy required is 16000 J.
42. Which is a device used to measure the amount of heat gained or lost by a substance that consists of a vessel made up of metals like copper or aluminium which are good conductors of heat and electricity?
- Thermometer
- Calorimeter
- Seismometer
- Thermostat
Explanation
A calorimeter is a device used to measure the amount of heat gained or lost by a substance. It consists of a vessel made up of metals like copper or aluminium which are good conductors of heat and electricity.
43. Which among the following statement is correct regarding calorimeter
- The metallic vessel is kept in an insulating jacket to prevent heat loss to the environment. There are two holes in it. Through one hole a thermometer is inserted to measure the temperature of the contents.
- A stirrer is inserted through another hole for stirring the content in the vessel. The vessel is filled with liquid which is heated by passing current through the heating element. Using this device, we can measure the heat capacity of the liquid in the container.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
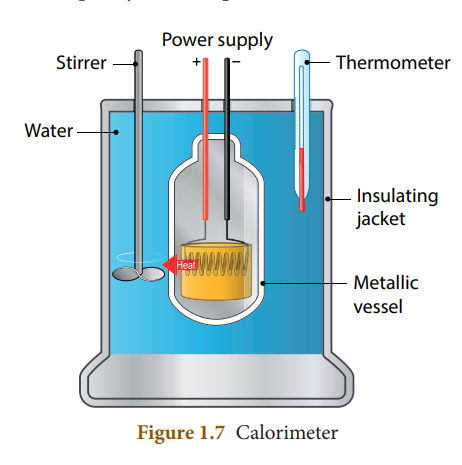
44. Which is a device which maintains the temperature of a place or an object constant?
- Thermometer
- Thermostat
- Thermo flask
- Hydrostat
Explanation
A thermostat is a device which maintains the temperature of a place or an object constant. The word thermostat is derived from two Greek words, ‘thermo’ meaning heat and ‘static’ meaning staying the same.
45. The world’s first ice-calorimeter was used in the year 1782 by whom?
- Joseph Fourier and Antoine Lavoisier
- Simeon Denis Poisson and Leonhard Euler
- Leonhard Euler and Joseph Fourier
- Antoine Lavoisier and Pierre Simon Laplace
Explanation
The world’s first ice-calorimeter was used in the year 1782 by Antoine Lavoisier and Pierre Simon Laplace, to determine the heat generated by various chemical changes.
46. Which among the following statement is correct regarding thermostat?
- Thermostats are used in any device or system that gets heated or cools down to a pre-set temperature. It turns an appliance or a circuit on or off when a particular temperature is reached.
- Devices which use thermostat include building heater, central heater in a room, air conditioner, water heater, as well as kitchen equipment’s including oven and refrigerators. Thermostat functions as the sensor of a thermal system not as a controller.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Devices which use thermostat include building heater, central heater in a room, air conditioner, water heater, as well as kitchen equipments including oven and refrigerators. Sometimes, a thermostat functions both as the sensor and the controller of a thermal system.
47. The Thermo Flask is also known as ____
- Flour flask
- Solid flask
- Vacuum flask
- Fourier flask
Explanation
The thermos flask (Vacuum flask) is an insulating storage vessel that keeps its content hotter or cooler than the surroundings for a longer time. It is primarily meant to enhance the storage period of a liquid by maintaining a uniform temperature and avoiding possibilities of getting a bad taste.
48. Which among the following statement is correct regarding Working of Thermos flask?
- A thermos flask has triple walls, which are evacuated. It is copper on the inside. The vacuum between the three walls prevents heat being transferred from the inside to the outside by conduction and convection.
- With very little air between the walls, there is almost no transfer of heat from the inner wall to the outer wall or vice versa. Conduction can only occur at the points where the two walls meet, at the top of the bottle and through an insulated support at the bottom. The silvered walls reflect radiated heat back to the liquid in the bottle.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
A thermos flask has double walls, which are evacuated. It is silvered on the inside. The vacuum between the two walls prevents heat being transferred from the inside to the outside by conduction and convection.

49. Which Scottish scientist invented Vacuum flask in 1892?
- Antoine Lavoisier
- Sir Thomas Bayes
- Frank Whittle
- Sir James Dewar
Explanation
The vacuum flask was invented by Scottish scientist Sir James Dewar in 1892. In his honour it is called Dewar flask. It’s also known as Dewar bottle.