10th Std Science Lesson Wise Questions in English – Part 1
10th Std Science Lesson Wise Questions in English
10th Science Lesson 1 Questions in English
1] Laws of Motion
1. Which of the following statement is correct?
- Some bodies are at rest and some are in motion.
- Things around us are related to one another
- There are various types of motion such as linear motion, circular motion, oscillatory motion, and so on
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Things around us are related to one another. Some bodies are at rest and some are in motion. Rest and motion are interrelated terms. There are various types of motion such as linear motion, circular motion, oscillatory motion, and so on.
2. Who proposed the laws of motion?
- Edison
- Newton
- Aristotle
- All the above
Explanation
Let us define force in a more scientific manner using the three laws proposed by Sir Isaac Newton. These laws help you to understand the motion of a body and also to predict the future course of its motion, if you know the forces acting on it. Before Newton formulated his three laws of motion, a different perception about the force and motion of bodies prevailed.
3. Which of the following statement is correct?
- Mechanics is the branch of physics that deals with the eff ect of force on bodies
- It is divided into two branches, namely, statics and dynamics.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
Mechanics is the branch of physics that deals with the effect of force on bodies. It is divided into two branches, namely, statics and dynamics.
4. __________ deals with the motion of bodies considering the cause of motion.
- Statics
- Kinematics
- Kinetics
- All the above
Explanation
Kinetics deals with the motion of bodies considering the cause of motion. Kinematics deals with the motion of bodies without considering the cause of motion.
5. _______ is rest under the action of forces.
- Kinetics
- Kinematics
- Statics
- Dynamics
Explanation
Statics deals with the bodies, which are at rest under the action of forces. Dynamics is the study of moving bodies under the action of forces. Dynamics is further divided into Kinematics and Kinetics.
6. According to_______ the natural state of earthly bodies is ‘rest’
- Edison
- Aristotle
- Galileo
- Einstein
Explanation
According to Aristotle a Greek Philosopher and Scientist, the natural state of earthly bodies is ‘rest’. He stated that a moving body naturally comes to rest without any external influence of the force. Such motions are termed as ‘natural motion’ (Force independent).
7. Which of the following statement about Aristotle is correct?
- Aristotle proposed that a force (a push or a pull) is needed to make the bodies to move from their natural state (rest)
- Violent motion is force independent
- He said, when two different mass bodies are dropped from a height, the lighter body falls faster than the heavier one.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Aristotle proposed that a force (a push or a pull) is needed to make the bodies to move from their natural state (rest) and behave contrary to their own natural state called as ‘violent motion’ (Force dependent). Further, he said, when two different mass bodies are dropped from a height, the heavier body falls faster than the lighter one.
8. Which of the following concepts were proposed by Galileo?
- Th e natural state of all earthly bodies is either the state of rest or the state of uniform motion
- A body in motion will continue to be in the same state of motion as long as no external force is applied
- When dropped from a height in vacuum, bodies of different size, shape and mass fall at the same rate and reach the ground at the same time.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Galileo proposed the following concepts about force, motion and inertia of bodies:
- Th e natural state of all earthly bodies is either the state of rest or the state of uniform motion.
- A body in motion will continue to be in the same state of motion as long as no external force is applied.
- When dropped from a height in vacuum, bodies of different size, shape and mass fall at the same rate and reach the ground at the same time.
9. The resistance applied by body to change in state is called as________
- Inertia
- Violent motion
- Gravity
- None
Explanation
When a force is applied on bodies, they resist any change in their state. This property of bodies is called ‘inertia’.
10. Assertion(A): While you are travelling in a bus or in a car, when a sudden brake is applied, the
upper part of your body leans in the forward direction
Reason(R): Anybody would like to continue to be in its state of rest or the state of motion. Th is
is known as ‘inertia’.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
While you are travelling in a bus or in a car, when a sudden brake is applied, the upper part of your body leans in the forward direction. Similarly, when the vehicle suddenly is move forward from rest, you lean backward. This is due to, anybody would like to continue to be in its state of rest or the state of motion. This is known as ‘inertia’. The inherent property of a body to resist any change in its state of rest or the state of uniform motion, unless it is influenced upon by an external unbalanced force, is known as ‘inertia’.
11. How many types of inertia are there?
- 4
- 3
- 5
- 2
Explanation
There are three types of inertia. They are Inertia of rest, Inertia of motion, Inertia of direction.
a) Inertia of rest: The resistance of a body to change its state of rest is called inertia of rest.
b) Inertia of motion: The resistance of a body to change its state of motion is called inertia of motion.
c) Inertia of direction: The resistance of a body to change its direction of motion is called inertia of direction
12. Take a glass tumbler and place a small cardboard on it. Then, flick the cardboard quickly. The
cardboard falls off the ground and the coin falls into the glass tumbler. This happens due to__
- Inertia of motion
- Inertia of rest
- Inertia of direction
- None
Explanation
In activity described above, the inertia of the coin keeps it in the state of rest when the cardboard moves. Then, when the cardboard has moved, the coin falls into the tumbler due to gravity. This happens due to ‘inertia of rest’.
13. Match the following:
- Inertia of motion 1. A sharp turn while driving a car
- Inertia of direction 2. An athlete runs some distance before jumping
- Inertia of rest 3. While vigorously shake the branches of a tree, some of the
leaves and fruits are detached and they fall down
- 3, 1, 2
- 1, 3, 2
- 2, 1, 3
- 2, 3, 1
Explanation
- An athlete runs some distance before jumping. Because, this will help him jump longer and higher. (Inertia of motion)
- When you make a sharp turn while driving a car, you tend to lean sideways, (Inertia of direction).
- When you vigorously shake the branches of a tree, some of the leaves and fruits are detached and they fall down, (Inertia of rest).
14. Which of the following results in increased impact of force?
- If velocity is more
- If mass of the body is more
- If velocity is less
- If mass is less
- 1, 4
- 2, 3
- 1, 2
- None
Explanation
The impact of a force is more if the velocity and the mass of the body is more. To quantify the impact of a force exactly, a new physical quantity known as linear momentum is defined.
15. The product of mass and velocity of a moving body gives________
- Magnitude of linear momentum
- Magnitude of circular momentum
- Acceleration
- Deceleration
Explanation
The product of mass and velocity of a moving body gives the magnitude of linear momentum. It acts in the direction of the velocity of the object.
Linear Momentum = mass × velocity
16. Which of the following statement is correct about Linear momentum?
- It is Scalar quantity
- It helps to measure the magnitude of a force
- Unit of momentum in SI system is kg m s–1
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Linear momentum is a vector quantity. It helps to measure the magnitude of a force. Unit of momentum in SI system is kg m s–1 and in C.G.S system its unit is g cm s-1.
17. Which law gives the definition of force as well as inertia?
- Newton’s 1st law
- Newton’s 2nd law
- Newton’s 3rd law
- None
Explanation
Newton’s First Law states that every – body continues to be in its state of rest or the state of uniform motion along a straight line unless it is acted upon by some external force. It gives the definition of force as well as inertia.
18. Which of the following statement about force is correct?
- Force has both magnitude and direction
- It is a vector quantity
- It produces or tries to produce the motion of a static body.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Force is an external effort in the form of push or pull, which:
1. produces or tries to produce the motion of a static body.
2. stops or tries to stop a moving body.
3. changes or tries to change the direction of motion of a moving body.
Force has both magnitude and direction. So, it is a vector quantity.
19. Which of the following statement is correct?
- Based on the direction in which the forces act, they can be classified into two types
- Two or more forces of equal or unequal magnitude acting along the same direction, parallel to each other are called like parallel forces.
- If two or more equal forces or unequal forces act along opposite directions parallel to each other, then they are called unlike parallel forces.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
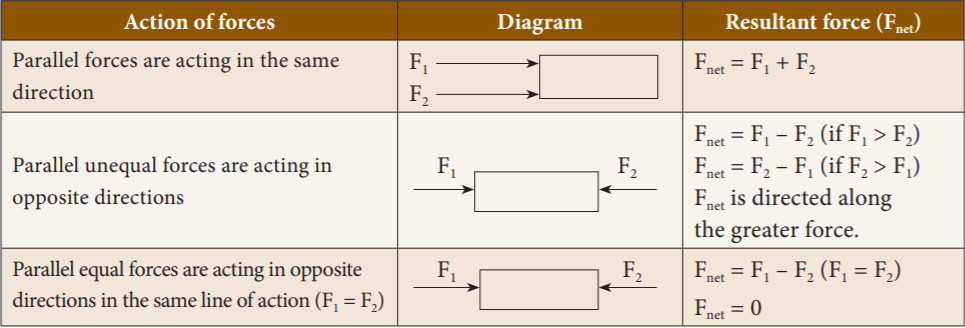
Based on the direction in which the forces act, they can be classified into two types as: (a) Like parallel forces and (b) Unlike parallel forces.
- Like parallel forces: Two or more forces of equal or unequal magnitude acting along the same direction, parallel to each other are called like parallel forces.
- Unlike parallel forces: If two or more equal forces or unequal forces act along opposite directions parallel to each other, then they are called unlike parallel forces.
20. Match the following:
- Parallel forces are acting in the same direction 1. F net = F1 + F2
- Parallel unequal forces are acting in opposite 2. F net = 0
directions
- Parallel equal forces are acting in 3. F net is directed along the greater force
opposite directions in the same line of action
- 3, 1, 2
- 1, 3, 2
- 1, 2, 3
- 2, 1, 3
Explanation
21. Which of the following statement is correct?
- When several forces act simultaneously on the same body, then the combined effect of the multiple forces can be represented by a single force, which is termed as ‘resultant force’
- It is equal to the vector sum (adding the magnitude of the forces with their direction) of all the forces
- Drawing water from a well is an example of balanced force
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
When several forces act simultaneously on the same body, then the combined effect of the multiple forces can be represented by a single force, which is termed as ‘resultant force’. It is equal to the vector sum (adding the magnitude of the forces with their direction) of all the forces. If the resultant force of all the forces acting on a body is equal to zero, then the body will be in equilibrium. Such forces are called balanced forces. If the resultant force is not equal to zero, then it causes the motion of the body due to unbalanced forces. Examples: Drawing water from a well, force applied with a crow bar, forces on a weight balance, etc.
22. The force used to bring system to equilibrium is called as__________
- Mediant
- Equilibrant
- Unbalanced force
- Balanced force
Explanation
A system can be brought to equilibrium by applying another force, which is equal to the resultant force in magnitude, but opposite in direction. Such force is called as ‘Equilibrant’.
23. Assertion(A): The door can be easily opened or closed when you apply the force at a point far
away from the fixed edge
Reason(R): This turning effect of the applied force is more when the distance between the fixed
edge and the point of application of force is more
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
The door can be easily opened or closed when you apply the force at a point far away from the fixed edge. In this case, the effect of the force you apply is to turn the door about the fixed edge. This turning effect of the applied force is more when the distance between the fixed edge and the point of application of force is more.
24. The rotating or turning effect of a force about a fixed point or fixed axis is called________
- Inertia
- Torque
- Momentum
- None
Explanation
The rotating or turning effect of a force about a fixed point or fixed axis is called moment of the force about that point or torque (τ).
25. Torque is equal to_______
- Force (F) X perpendicular distance (d)
- Force / perpendicular distance
- Mass X velocity
- Mass / velocity
Explanation
Torque is measured by the product of the force (F) and the perpendicular distance (d) between the fixed point or the fixed axis and the line of action of the force. τ = F × d
26. Which of the following statement about Torque is correct?
- Torque is a vector quantity
- It is acting along the direction, perpendicular to the plane containing the line of action
- Its SI unit is N/m
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Torque is a vector quantity. It is acting along the direction, perpendicular to the plane containing the line of action of force and the distance. Its SI unit is N m.
27. Which of the following statement is correct?
- Two equal and unlike parallel forces applied simultaneously at two distinct points constitute a couple
- Rotating effect of a couple is known as moment of a couple.
- It does not produce any translatory motion since the resultant is zero
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Two equal and unlike parallel forces applied simultaneously at two distinct points constitute a couple. The line of action of the two forces does not coincide. It does not produce any translatory motion since the resultant is zero. But, a couple results in causes the rotation of the body. Rotating effect of a couple is known as moment of a couple.
28. What is the CGS unit of moment of Couple?
- N m
- N
- dyne cm
- dyne
Explanation
Moment of a couple is measured by the product of any one of the forces and the perpendicular distance between the line of action of two forces. The turning effect of a couple is measured by the magnitude of its moment. M = F × S
The unit of moment of a couple is newton metre (N m) in SI system and dyne cm in CGS system.
29. By convention, the direction of moment of a force is taken as___ if the body is rotated in the___
direction
- Positive, Clockwise
- Negative, Anti-Clockwise
- Positive, Anti-clockwise
- Negative, Clockwise
Explanation
By convention, the direction of moment of a force or couple is taken as positive if the body is rotated in the anti-clockwise direction and negative if it is rotate in the clockwise direction.
30. In which of the following involves the Application of Torque?
- Gears
- Sea-saw
- Steering Wheel
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Application of Torque:
- A gear is a circular wheel with teeth around its rim. It helps to change the speed of rotation of a wheel by changing the torque and helps to transmit power.
- Most of you have played on the sea-saw. Since there is a difference in the weight of the persons sitting on it, the heavier person lifts the lighter person. When the heavier person comes closer to the pivot point (fulcrum) the distance of the line of action of the force decreases. It causes less amount of torque to act on it. This enables the lighter person to lift the heavier person.
- A small steering wheel enables you to manoeuore a car easily by transferring a torque to the wheels with less effort.
31. Which of the following statement regarding Principle of Moments is correct?
- When a number of like or unlike parallel forces act on a rigid body and the body is in equilibrium, then the algebraic sum of the moments in the clockwise direction is equal to the algebraic sum of the moments in the anticlockwise direction.
- The algebraic sum of the moments of all the individual forces about any point is equal to positive.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
When a number of like or unlike parallel forces act on a rigid body and the body is in equilibrium, then the algebraic sum of the moments in the clockwise direction is equal to the algebraic sum of the moments in the anticlockwise direction. In other words, at equilibrium, the algebraic sum of the moments of all the individual forces about any point is equal to zero.
32. Which law helps us to measure the amount of force?
- Newton’s 1st law
- Newton’s 2nd law
- Newton’s 3rd law
- None
Explanation
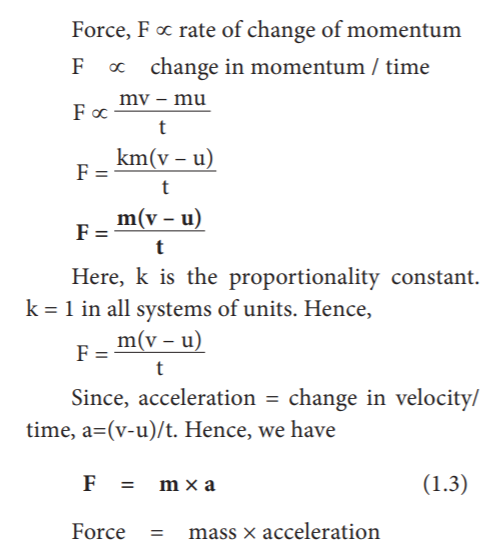
According to Newton’s Second law, “the force acting on a body is directly proportional to the rate of change of linear momentum of the body and the change in momentum takes place in the direction of the force”. This law helps us to measure the amount of force. So, it is also called as ‘law of force’
33. Which of the following statement is correct?
- Force ∝ rate of change of momentum
- F ∝ change in momentum
- F inversely proportionally to time
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
34. Which of the following statement is correct?
- Force is required to produce the acceleration of a body
- In a uniform circular motion, even though the speed (magnitude of velocity) remains constant, the direction of the velocity changes at every point on the circular path
- The force, which produces this acceleration is called as centrifugal force
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Force is required to produce the acceleration of a body. In a uniform circular motion, even though the speed (magnitude of velocity) remains constant, the direction of the velocity changes at every point on the circular path. So, the acceleration is produced along the radius called as centripetal acceleration. The force, which produces this acceleration is called as centripetal force
35. What is the CGS unit of Force?
- Newton
- dyne
- Kg m s
- Kg m s-1
Explanation
Units of force: SI unit of force is newton (N) and in C.G.S system its unit is dyne. Definition of 1 newton (N): The amount of force required for a body of mass 1 kg produces an acceleration of 1 m s –2, 1 N = 1 kg m s –2.
36. 1 Newton =
- 10^3 dyne
- 10^5 dyne
- 10^2 dyne
- 10^7 dyne
Explanation
The amount of force required for a body of mass 1 gram produces an acceleration of 1 cm s –2, 1 dyne = 1 g cm s -2; also 1 N = 10^5 dyne.
37. A large force acting for a very short interval of time is called_______
- Inertia
- Impulse
- Couple
- None
Explanation
A large force acting for a very short interval of time is called as ‘Impulsive force’. When a force F acts on a body for a period of time t, then the product of force and time is known as ‘impulse’ represented by ‘J’
Impulse, J = F × t
38. What is the unit of Impulse?
- Newton
- N s
- N m
- dyne
Explanation
Impulse, J = F × t
By Newton’s second law F = Δ p / t (Δ refers to change),
Δ p = F × t
Impulse is also equal to the magnitude of change in momentum. Its unit is kg m s-1 or N s.
39. Assertion(A): Automobiles are fitted with springs and shock absorbers to reduce jerks while
moving on uneven roads
Reason(R): A smaller force acting for a longer period of time is one of the change in Momentum.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
Given amount of change in momentum can be achieved in two ways. They are:
i. a large force acting for a short period of time and
ii. a smaller force acting for a longer period of time.
Examples of inertia:
- Automobiles are fitted with springs and shock absorbers to reduce jerks while moving on uneven roads.
- In cricket, a fielder pulls back his hands while catching the ball. He experiences a smaller force for a longer interval of time to catch the ball, resulting in a lesser impulse on his hands
40. Which of the following equation show NEWTON’S THIRD LAW OF MOTION?
- F net = Fa + Fb
- F net = Fa – Fb
- Fb = –Fa
- Fb = Fa
Explanation
Newton’s third law states that ‘for every action, there is an equal and opposite reaction. They always act on two different bodies. If a body A applies a force FA on a body B, then the body B reacts with force FB on the body A, which is equal to FA in magnitude, but opposite in direction.
Fb = –Fb.
41. Which of the following are the examples of Newton’s third law of motion?
- When a person swims, he pushes the water using the hands backwards, and the water pushes the swimmer in the forward direction.
- When birds fly, they push the air downwards with their wings and the air pushes the bird upwards.
- When we turn the turn the steering wheel, the wheel turns
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Examples Newton’s third law of Motion:
- When birds fly, they push the air downwards with their wings (Action) and the air pushes the bird upwards (Reaction).
- When a person swims, he pushes the water using the hands backwards (Action), and the water pushes the swimmer in the forward direction (Reaction).
- When you fire a bullet, the gun recoils backward and the bullet is moving forward (Action) and the gun equalises this forward action by moving backward (Reaction).
42. Which of the following statement is correct?
- There is no change in the linear momentum of a system of bodies as long as no net external force acts on them
- There is no change in the linear momentum of a system of bodies as long as a external force acts on them
- There is no change in the linear momentum of a system of bodies as long as no net internal force acts on them
- None
Explanation
There is no change in the linear momentum of a system of bodies as long as no net external force acts on them.
m1v1 + m2v2 = m1u1 + m2u2
The above equation confirms in the absence of an external force, the algebraic sum of the momentum after collision is numerically equal to the algebraic sum of the momentum before collision.
43. Propulsion of rockets is based on_________
- Newton’s 1st law
- Newton’s 2nd law
- Newton’s 3rd law
- None
Explanation
Propulsion of rockets is based on the law of conservation of linear momentum as well as Newton’s III law of motion.
44. Which of the following statement is correct?
- Rockets are filled with a fuel (only liquid) in the propellant tank
- When the rocket is fired, this fuel is burnt and a hot gas is ejected with a high speed from the nozzle of the rocket, producing a huge momentum.
- To balance this momentum, an equal and opposite reaction force is produced in the combustion chamber, which makes the rocket project forward
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Rockets are filled with a fuel (either liquid or solid) in the propellant tank. When the rocket is fired, this fuel is burnt and a hot gas is ejected with a high speed from the nozzle of the rocket, producing a huge momentum. To balance this momentum, an equal and opposite reaction force is produced in the combustion chamber, which makes the rocket project forward.
45. When rocket moves the mass of the rocket_____
- Stays constant
- Increases
- Decreases
- None
Explanation
While in motion, the mass of the rocket gradually decreases, until the fuel is completely burnt out. Since, there is no net external force acting on it, the linear momentum of the system is conserved.
46. The mass of the rocket decreases with______
- Temperature
- Altitude
- Fuel
- All the above
Explanation
The mass of the rocket decreases with altitude, which results in the gradual increase in velocity of the rocket. At one stage, it reaches a velocity, which is sufficient to just escape from the gravitational pull of the Earth. This velocity is called escape velocity.
47. Which of the following statement is correct about Newton’s universal law of gravitation?
- This law states that every particle of matter in this universe attracts every other particle with a force
- This law is proportional to the square of the distance between the centre of these masses
- This force is directly proportional to the product of their masses
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Newton’s universal law of gravitation states that every particle of matter in this universe attracts every other particle with a force. This force is directly proportional to the product of their masses and inversely proportional to the square of the distance between the centres of these masses. The direction of the force acts along the line joining the masses.
48. Force between the masses is______
- Attractive
- Repulsive
- Either a or b
- None
Explanation
Force between the masses is always attractive and it does not depend on the medium where they are placed.
49. What is the universal gravitational constant?
- 6.674 × 10^–11 N m^2 kg^–2
- 6.674 × 10–11 N m^2 kg^2
- 9.8 m/s
- 9.8 m s
Explanation
Let, m1 and m2 be the masses of two bodies A and B placed r metre apart in space,
Force,
F ∝ m1 × m2
F ∝ 1/ r2
F = G m1 m2/ r^2
Where G is the universal gravitational constant. Its value in SI unit is
6.674 × 10–11 N m^2 kg^–2.
50. When we throw an object upwards, while it returns down________
- Velocity of the object keeps changing as it falls down
- Velocity will be constant
- Initially increase then decrease constant
- None
Explanation
The velocity of the object keeps changing as it falls down. This change in velocity must be due to the force acting on the object. The acceleration of the body is due to the Earth’s gravitational force. So, it is called as ‘acceleration due to the gravitational force of the Earth’ or ‘acceleration due to gravity of the Earth’. It is represented as ‘g’. Its unit is m s ^–2.
51. Mean value of the acceleration due to gravity is taken as________ m/s^2
- 10
- 9.8
- 9.6
- 8.6
Explanation
Mean value of the acceleration due to gravity is taken as 9.8 m s ^–2 on the surface of the Earth. This means that the velocity of a body during the downward free fall motion varies by 9.8 m s^–1 for every 1 second. However, the value of ‘g’ is not the same at all points on the surface of the earth.
52. What is the approximate radius of earth?
- 9400 km
- 6400 km
- 4600 km
- 3600 km
Explanation
The radius of the Earth is R =6378 km = 6400 km approximately. By Newton’s law of gravitation, the force acting on the body is given by,
F = G M m/ R^2.
53. What is the equation for Acceleration due to gravity?
- GM / R^2
- GM
- GM R^2
- R^2 / GM
Explanation
According to Newton’s second law of motion, the force acting on the body is given by the product of its mass and acceleration (called as weight). Here, acceleration of the body is under the action of gravity hence a = g.
F = m a = m g
Acceleration due to gravity g = GM / R^2
54. What is the mass of Earth(kg)?
- 5.972 × 10^24
- 5.972 × 10^37
- 5.972 × 10^20
- 5.972 × 10^16
Explanation
Mass of the Earth M = g R^2 /G
Substituting the known values of g, R and G,
you can calculate the mass of the Earth as M = 5.972 × 10^24 kg
55. Which of the following statemen is correct?
- Geometric radius of the Earth is maximum in the equatorial region
- Geometric radius of the Earth is minimum in the equatorial region
- The value of g is maximum in the polar region and minimum at the equatorial region
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Since, g depends on the geometric radius of the Earth, (g ∝ 1/R^2), its value changes from one place to another on the surface of the Earth. Since, the geometric radius of the Earth is maximum in the equatorial region and minimum in the polar region, the value of g is maximum in the polar region and minimum at the equatorial region.
56. When you move to a higher altitude from the surface of the Earth, the value of g______
- Increases
- Decreases
- Initially increase then decrease
- Initially decrease then increase
Explanation
When you move to a higher altitude from the surface of the Earth, the value of g reduces. In the same way, when you move deep below the surface of the Earth, the value of g reduces. Value of g is zero at the centre of the Earth.
57. Which of the following statement is correct?
- Mass of a body is defined as the quantity of matter contained in the body
- Its SI unit is kilogram (kg)
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
Mass is the basic property of a body. Mass of a body is defined as the quantity of matter contained in the body. Its SI unit is kilogram (kg).
58. Which of the following statement is correct?
- Weight of a body is defined as the gravitational force exerted on it due to the Earth’s gravity alone
- Weight is a Scalar quantity
- SI unit of weight is newton
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Weight is a vector quantity. Direction of weight is always towards the centre of the Earth. SI unit of weight is newton (N). Weight of a body varies from one place to another place on the Earth since it depends on the acceleration due to gravity of the Earth (g), which is not the same at all places on the Earth.
59. Which of the following remains constant in Earth and moon?
- Mass
- Weight
- Both a and b
- None
Explanation
The value of acceleration due to gravity on the surface of the moon is 1.625 ms–2. This is about 0.1654 times the acceleration due to gravity of the Earth. If a person whose mass is 60 kg stands on the surface of Earth, his weight would be 588 N (W= mg = 60 × 9.8). If the same person goes to the surface of the Moon, he would weigh only 97.5 N (W = 60 × 1.625). But, his mass remains the same (60 kg) on both the Earth and the Moon.
60. Which of the following statement is incorrect?
- The weight that you feel to possess is not the same as your actual weight called apparent weight.
- Apparent weight is the weight of the body acquired due to the action of gravity and other external forces acting on the body
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
The weight that you feel to possess is not the same as your actual weight called apparent weight. Apparent weight is the weight of the body acquired due to the action of gravity and other external forces acting on the body.
61. Match the following:
- Lift is moving upward 1. Apparent weight is equal to the actual weight
- Lift is moving downward 2. Apparent weight is equal to zero
- Lift is at rest 3. Apparent weight is greater than the actual weight
- Lift is falling down freely 4. Apparent weight is lesser than the actual weight
- 3, 2, 1, 4
- 2, 1, 3, 4
- 3, 4, 1, 2
- 3, 2, 4, 1
Explanation

62. Which of the following statement is correct?
- We believe that the astronauts in the orbiting space station do not experience any gravitational force of the Earth
- But this is absolutely correct
- Astronauts are not floating but falling freely around the earth due to their huge orbital velocity.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Some of us believe that the astronauts in the orbiting space station do not experience any gravitational force of the Earth. So, they float. But this is absolutely wrong. Astronauts are not floating but falling freely around the earth due to their huge orbital velocity. Since space station and astronauts have equal acceleration, they are under free fall condition. Hence, both the astronauts and the space station are in the state of weightlessness.
63. Which of the following statement is correct?
- Dimensions of the heavenly bodies can be measured using the gravitation law
- Newton’s law of gravitation helps in discovering new stars and planets.
- It helps to predict the path of the astronomical bodies
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Application of Newton’s law of gravitation:
1) Dimensions of the heavenly bodies can be measured using the gravitation law. Mass of the Earth, radius of the Earth, acceleration due to gravity, etc. can be calculated with a higher accuracy.
2) Helps in discovering new stars and planets.
3) One of the irregularities in the motion of stars is called ‘Wobble’ leads to the disturbance in the motion of a planet nearby. In this condition the mass of the star can be calculated using the law of gravitation.
4) Helps to explain germination of roots is due to the property of geotropism which is the property of a root responding to the gravity.
5) Helps to predict the path of the astronomical bodies.
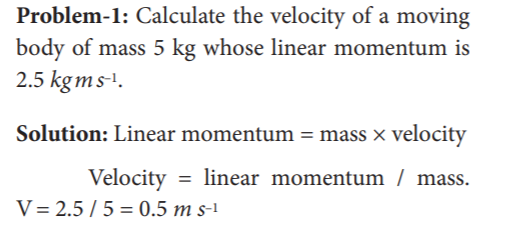
64. Calculate the velocity of a moving body of mass 5 kg whose linear momentum is 2.5 kg m s–1.
- 0.5 m s^–1
- 5 m s^–1
- 1 m s^–1
- 50 m s^–1
Explanation
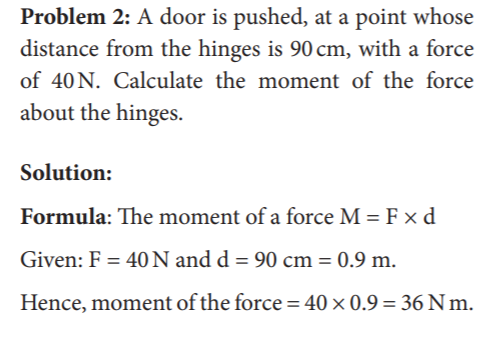
65. A door is pushed, at a point whose distance from the hinges is 90 cm, with a force of 40N.
Calculate the moment of the force about the hinges
- 36 Nm
- 36 N
- 63 Nm
- 63 N/m
Explanation
10th Science Lesson 2 Questions in English
2] Optics
1. Which of the following statement is correct?
- Light is a form of energy which travels in the form of waves
- The path of light is called ray of light and group of these rays are called as beam of light.
- Any object which gives out light are termed as source of light and is called as luminous objects
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Light is a form of energy which travels in the form of waves. The path of light is called ray of light and group of these rays are called as beam of light. Any object which gives out light are termed as source of light. Some of the sources emit their own light and they are called as luminous objects. All the stars, including the Sun, are examples for luminous objects.
2. Which of the following statement about properties of light is correct?
- Light is a form of energy.
- Light always travels in a Zig Zag manner
- When light is incident on the interface between two media, it is partly reflected and partly refracted
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
PROPERTIES OF LIGHT:
- Light is a form of energy
- Light always travels along a straight line.
- When light is incident on the interface between two media, it is partly reflected and partly refracted.
- The speed of light can be calculated using the following equation: c = ν λ (c – velocity of light).
3. Assertion(A): Light can even travel through vacuum.
Reason(R): Light does not need any medium for its propagation.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
Light does not need any medium for its propagation. It can even travel through vacuum. The speed of light in vacuum or air is, c = 3 × 10^8 m/s. Since, light is in the form of waves, it is characterized by a wavelength (λ) and a frequency (ν), which are related by the following equation: c = ν λ (c – velocity of light).
4. Which of the following light has lowest wavelength?
- Green
- Red
- Violet
- Yellow
Explanation
Different coloured light has different wavelength and frequency. Among the visible light, violet light has the lowest wavelength and red light has the highest wavelength.
5. The deviation of ray of light is called_____
- Reflection
- Depression
- Refraction
- None
Explanation
When a ray of light travels from one transparent medium into another obliquely, the path of the light undergoes deviation. This deviation of ray of light is called refraction.
6. Which of the following statement is correct?
- The velocity of light is more in a rarer medium and less in a denser medium
- The velocity of light is less in a rarer medium and more in a denser medium
- The velocity of light is same in both rarer medium and in denser medium
- None
Explanation
Refraction takes place due to the difference in the velocity of light in different media. The velocity of light is more in a rarer medium and less in a denser medium. Refraction of light obeys two laws of refraction.
7. According to First law of refraction which of the following lie in the same plane?
- Incident ray
- Reflected ray
- Refracted ray
- Normal
- 1, 3, 4
- 2, 3, 4
- 1, 2, 4
- All the above
Explanation
First law of refraction:
According to first law of refraction, the incident ray, the refracted ray of light and the normal to the refracting surface all lie in the same plane.
8. Which of the following statement is incorrect?
- Second law of refraction is also known as Snell’s law
- Refractive index gives us an idea of how fast or how slow light travels in a medium
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
Second law of refraction:
The ratio of the sine of the angle of incidence and sine of the angle of refraction is equal to the ratio of refractive indices of the two media. This law is also known as Snell’s law. Refractive index gives us an idea of how fast or how slow light travels in a medium. The ratio of speed of light in vacuum to the speed of light in a medium is defined as refractive index ‘µ’ of that medium.
9. The speed of light in a medium is___ if the refractive index of the medium is____
- High, Low
- Low, High
- Low, low
- Both a and b
Explanation
The speed of light in a medium is low if the refractive index of the medium is high and vice versa. The above statement is drawn out of Second law of refraction or Snell’s law.
10. Which of the following statement is incorrect?
- When light travels from a denser medium into a rarer medium, the refracted ray is bent towards from the normal drawn to the interface
- When light travels from a rarer medium into a denser medium, the refracted ray is bent away from the normal drawn to the interface.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
- When light travels from a denser medium into a rarer medium, the refracted ray is bent away from the normal drawn to the interface.
- When light travels from a rarer medium into a denser medium, the refracted ray is bent towards the normal drawn to the interface.
11. Assertion(A): Sun is the fundamental and natural source of light
Reason(R): It is a monochromatic source of light
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
We know that Sun is the fundamental and natural source of light. If a source of light produces a light of single colour, it is known as a monochromatic source.
12. Which of the following is an example of composite source of light?
- Sun
- Sodium vapour lamp
- Mercury vapour lamp
- 1 alone
- 1, 2
- 1, 3
- None
Explanation
A composite source of light produces a white light which contains light of different colours. Sun light is a composite light which consists of light of various colours or wavelengths. Another example for a composite source is a mercury vapour lamp.
13. Which of the following statement is correct?
- When a beam of white light is refracted through any transparent media such as glass it is split into its component colours
- This phenomenon is called as ‘dispersion of light’.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
When a beam of white light or composite light is refracted through any transparent media such as glass or water, it is split into its component colours. This phenomenon is called as ‘dispersion of light’. The band of colours is termed as spectrum.
14. Arrange the spectrum of colours in order
- Indigo
- Yellow
- Red
- Violet
- 1, 3, 2, 4
- 4, 1, 2, 3
- 4, 1, 3, 2
- 2, 1, 3, 4
Explanation
The band of colours is termed as spectrum. This spectrum consists of following colours: Violet, Indigo, Blue, Green, Yellow, Orange, and Red. These colours are represented by the acronym “VIBGYOR”.
15. Why do we get the spectrum when white light is refracted by a transparent medium?
- Bending of the light at different angles
- Converging of light
- Bending of light at same angle
- All the above
Explanation
We get the spectrum when white light is refracted by a transparent medium, this is because different coloured lights are bent through different angles. That is the angle of refraction is different for different colours.
16. Which colour has highest angle of refraction?
- Blue
- Red
- Violet
- Orange
Explanation
Angle of refraction is the smallest for red and the highest for violet. From Snell’s law, we know that the angle of refraction is determined in terms of the refractive index of the medium. Hence, the refractive index of the medium is different for different coloured lights. This indicates that the refractive index of a medium is dependent on the wavelength of the light.
17. Which of the following statement is correct?
- When sunlight enters the Earth’s atmosphere, the atoms and molecules of different gases present in the atmosphere refract the light in all possible directions
- This is called as ‘Scattering of light’.
- The interacting particle of the medium is called as ‘scatterer’.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
When sunlight enters the Earth’s atmosphere, the atoms and molecules of different gases present in the atmosphere refract the light in all possible directions. This is called as ‘Scattering of light’. In this phenomenon, the beam of light is redirected in all directions when it interacts with a particle of medium. The interacting particle of the medium is called as ‘scatterer’.
18. Based on initial and final energy of the light beam, scattering can be classified into_______ types
- 4
- 2
- 3
- 5
Explanation
When a beam of light, interacts with a constituent particle of the medium, it undergoes many kinds of scattering. Based on initial and final energy of the light beam, scattering can be classified as, 1) Elastic scattering 2) Inelastic scattering
19. Which of the following statement is correct?
- If the energy of the incident beam of light and the scattered beam of light are same, then it is called as ‘elastic scattering’.
- If the energy of the incident beam of light and the scattered beam of light are different, then it is called as ‘elastic scattering’.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
If the energy of the incident beam of light and the scattered beam of light are same, then it is called as ‘elastic scattering’.
20. Nature and size of the scatterer results in_______ types of scattering
- Rayleigh scattering
- Mie scattering
- Raman scattering
- Tyndall scattering
- 1, 2, 4
- 2, 3, 4
- 1, 3, 4
- All the above
Explanation
If the energy of the incident beam of light and the scattered beam of light are not same, then it is called as ‘inelastic scattering’. The nature and size of the scatterer results in different types of scattering. They are:
- Rayleigh scattering
- Mie scattering
- Tyndall scattering
- Raman scattering
21. The scattering of sunlight by the atoms or molecules of the gases in the earth’s atmosphere is____
- Rayleigh scattering
- Raman scattering
- Mie scattering
- Tyndall scattering
Explanation
The scattering of sunlight by the atoms or molecules of the gases in the earth’s atmosphere is known as Rayleigh scattering.
22. Which of the following expresses the Amount of scattering according to Rayleigh scattering?
- 1 /λ^4
- 1 /λ^3
- 1 /λ^2
- 1 /λ^7
Explanation
Rayleigh’s scattering law states that, “The amount of scattering of light is inversely proportional to the fourth power of its wavelength”.
Amount of scattering ‘S’ ∝ 1 /λ^4
23. Assertion(A): Scattering causes the sky to appear in blue colour
Reason(R): When sunlight passes through the atmosphere, the blue colour (shorter wavelength)
is scattered to a greater extent than the red colour (longer wavelength).
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
According to Rayleigh’s scattering law, the shorter wavelength colours are scattered much more than the longer wavelength colours. When sunlight passes through the atmosphere, the blue colour (shorter wavelength) is scattered to a greater extent than the red colour (longer wavelength). This scattering causes the sky to appear in blue colour.
24. Assertion(A): At sunrise and sunset, the light rays from the Sun appears red in colour
Reason(R): Most of the blue lights are scattered away and only the red light which gets least
scattered reaches us during morning and the evening
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
At sunrise and sunset, the light rays from the Sun have to travel a larger distance in the atmosphere than at noon. Hence, most of the blue lights are scattered away and only the red light which gets least scattered reaches us. Therefore, the colour of the Sun is red at sunrise and sunset.
25. Which of the following statement is correct about Mie scattering?
- Mie scattering takes place when the diameter of the scatterer is similar to or larger than the wavelength of the incident light.
- The amount of scattering is dependents on the wave length.
- Mie scattering is caused by pollen, dust, smoke, water droplets, and other particles in the lower portion of the atmosphere
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Mie scattering takes place when the diameter of the scatterer is similar to or larger than the wavelength of the incident light. It is also an elastic scattering. The amount of scattering is independent of wave length. Mie scattering is caused by pollen, dust, smoke, water droplets, and other particles in the lower portion of the atmosphere.
26. __________ is responsible for the white appearance of the cloud
- Rayleigh scattering
- Mie scattering
- Tyndall scattering
- Raman scattering
Explanation
Mie scattering is responsible for the white appearance of the clouds. When white light falls on the water drop, all the colours are equally scattered which together form the white light.
27. When a beam of sunlight, enters into a dusty room through a window, then its path becomes
visible to us. This an example of____ effect
- Rayleigh scattering
- Mie scattering
- Tyndall scattering
- Raman scattering
Explanation
When a beam of sunlight, enters into a dusty room through a window, then its path becomes visible to us. This is because, the tiny dust particles present in the air of the room scatter the beam of light. This is an example of Tyndall Scattering.
28. Tyndall Effect takes place in______ solution
- Colloidal
- True
- Suspension
- All the above
Explanation
The scattering of light rays by the colloidal particles in the colloidal solution is called Tyndall Scattering or Tyndall Effect. Colloid is a microscopically small substance that is equally dispersed throughout another material. Example: Milk, Ice cream, muddy water, smoke.
29. Which scattering speaks about additional frequencies in the scattering the monochromatic light?
- Rayleigh scattering
- Mie scattering
- Tyndall scattering
- Raman scattering
Explanation
When a parallel beam of monochromatic (single coloured) light passes through a gas or liquid or transparent solid, a part of light rays is scattered. The scattered light contains some additional frequencies (or wavelengths) other than that of incident frequency (or wavelength). This is known as Raman scattering or Raman Effect.
30. The spectral lines having frequency equal to the incident ray frequency is_____
- Normal line
- Rayleigh line
- Incident line
- None
Explanation
Raman Scattering is defined as “The interaction of light ray with the particles of pure liquids or transparent solids, which leads to a change in wavelength or frequency.” The spectral lines having frequency equal to the incident ray frequency is called ‘Rayleigh line’ and the spectral lines which are having frequencies other than the incident ray frequency are called ‘Raman lines’.
31. The lines having frequencies higher than the incident frequency are called as_____
- Stoke lines
- Anti-stoke lines
- Refracted lines
- All the above
Explanation
The lines having frequencies lower than the incident frequency is called stokes lines and the lines having frequencies higher than the incident frequency are called Anti-stokes lines.
32. How many spherical refracting surfaces are there in lenses?
- 1
- 4
- 3
- 2
Explanation
A lens is an optically transparent medium bounded by two spherical refracting surfaces or one plane and one spherical surface.
33. Which of the following are the features of Convex lens?
- It is a lens bounded by two spherical surfaces such that it is thicker at the centre than at the edges
- A convex lens is also called as converging lens
- A beam of light passing through it, is converged to a point
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Convex or bi-convex lens is a lens bounded by two spherical surfaces such that it is thicker at the centre than at the edges. A beam of light passing through it, is converged to a point. So, a convex lens is also called as converging lens.
34. Which of the following statement is correct about Concave or bi-concave Lens?
- It is a lens bounded by two spherical surfaces such that it is thinner at the centre than at the edges
- A parallel beam of light passing through it will spread in
- A concave lens is also called as diverging lens.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Concave or bi-concave Lens is a lens bounded by two spherical surfaces such that it is thinner at the centre than at the edges. A parallel beam of light passing through it, is diverged or spread out. So, a concave lens is also called as diverging lens.
35. If one of the faces of a bi-convex lens is plane, it is known as a_______
- Plano-concave lens
- Convex mirror
- Plano-convex lens
- None
Explanation
Plano-convex lens: If one of the faces of a bi-convex lens is plane, it is known as a planoconvex lens. Plano-concave lens: If one of the faces of a bi-concave lens is plane, it is known as a planoconcave lens
36. Which of the following shows the features of image formed?
- Position
- Size
- Nature of the image
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
When an object is placed in front of a lens, the light rays from the object fall on the lens. The position, size and nature of the image formed can be understood only if we know certain basic rules.
37. Match the following with
- Ray of light strikes at the optical centre 1. refracted ray will be parallel to the
principal axis
- Ray of light parallel to principal axis 2. it continues to follow its path without
any deviation
- Ray of light strikes directed towards 3. Either converge or diverge from principal
the principal focus axis based on type of lens
- 3, 1, 2
- 2, 3, 1
- 1, 3, 2
- 2, 1, 3
Explanation
Rule-1: When a ray of light strikes the convex or concave lens obliquely at its optical centre, it continues to follow its path without any deviation
Rule-2: When rays parallel to the principal axis strikes a convex or concave lens, the refracted rays are converged to (convex lens) or appear to diverge from (concave lens) the principal focus
Rule-3: When a ray passing through (convex lens) or directed towards (concave lens) the principal focus strikes a convex or concave lens, the refracted ray will be parallel to the principal axis
38. What will be the size of the image in convex lens in case of Object at infinity?
- Size of the image is much smaller than that of the object
- Size of the image is equal to that of the object
- Size of the image is much larger than that of the object
- None
Explanation
Let us discuss the formation of images by a convex lens when the object is placed at various positions. When an object is placed at infinity, a real image is formed at the principal focus. The size of the image is much smaller than that of the object.
39. Match the following with the position of image formation in the case of convex lens
- Object placed beyond C 1. real image is formed at infinity
- Object placed at C 2. real and inverted image is formed behind
the centre of curvature
- Object placed between F and C 3. image is formed between the centre of
curvature and the principal focus
- Object placed at the principal focus F 4. a real and inverted image is formed at the
other centre of curvature
- 1, 2, 4, 3
- 3, 4, 2, 1
- 3, 2, 1, 4
- 2, 1, 4, 3
Explanation
Object placed beyond C (>2F): When an object is placed behind the centre of curvature (beyond C), a real and inverted image is formed between the centre of curvature and the principal focus. Th e size of the image is the same as that of the object
Object placed at C: When an object is placed at the centre of curvature, a real and inverted image is formed at the other centre of curvature. The size of the image is the same as that of the object.
Object placed between F and C: When an object is placed in between the centre of curvature and principal focus, a real and inverted image is formed behind the centre of curvature. The size of the image is bigger than that of the object
Object placed at the principal focus F: When an object is placed at the focus, a real image is formed at infinity. The size of the image is much larger than that of the object
Object placed between the principal focus F and optical centre O: When an object is placed in between principal focus and optical centre, a virtual image is formed. The size of the image is larger than that of the object
40. Which of the following are the uses of Convex lenses?
- Convex lenses are used as magnifying lenses
- They are used as camera lenses
- They are used to correct the defect of vision called myopia
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
APPLICATIONS OF CONVEX LENSES:
1. Convex lenses are used as camera lenses
2. They are used as magnifying lenses
3. They are used in making microscope, telescope and slide projectors
4. They are used to correct the defect of vision called hypermetropia
41. Which of the following statement about concave lens is incorrect?
- When an object is placed at infinity, a virtual image is formed at the focus
- When an object is placed at a finite distance from the lens, a virtual image is formed between optical centre and focus of the concave lens
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
When an object is placed at infinity, a virtual image is formed at the focus. The size of the image is much smaller than that of the object. When an object is placed at a finite distance from the lens, a virtual image is formed between optical centre and focus of the concave lens. The size of the image is smaller than that of the object.
42. What happens the distance between the object and the lens is decreased in a Concave lens?
- The distance between the image and the lens also keeps decreases
- The distance between the image and the lens also keeps increases
- The distance between the image and the lens will be same
- None
Explanation
In a concave lens, as the distance between the object and the lens is decreased, the distance between the image and the lens also keeps decreasing. Further, the size of the image formed increases as the distance between the object and the lens is decreased.
43. Which of the following are the uses of concave lens?
- Concave lenses are used as eye lens of ‘Galilean Telescope’
- They are used in wide angle spy hole in doors
- They are used to correct the defect of vision called ‘myopia’
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
APPLICATIONS OF CONCAVE LENSES:
1. Concave lenses are used as eye lens of ‘Galilean Telescope’
2. They are used in wide angle spy hole in doors.
3. They are used to correct the defect of vision called ‘myopia’
44. Which of the following gives the focal length (f) of the lens?
- u + v
- u – v
- 1/u – 1/v
- 1/u + 1/v
Explanation
Like spherical mirrors, we have lens formula for spherical lenses. The lens formula gives the relationship among distance of the object (u), distance of the image (v) and the focal length (f) of the lens. It is expressed as:
1/f = 1/u – 1/v
It is applicable to both convex and concave lenses. We need to give an at most care while solving numerical problems related to lenses in taking proper signs of different quantities.
45. Which of the following statement about Cartesian sign conventions are correct?
- The object is always placed on the left side of the lens.
- The distances measured upward and perpendicular to the principal axis is taken as positive
- The distances measured in the same direction as that of incident light are taken as positive.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Cartesian sign conventions are used for measuring the various distances in the ray diagrams of spherical lenses. According to cartesian sign convention,
1. The object is always placed on the left side of the lens.
2. All the distances are measured from the optical centre of the lens.
3. The distances measured in the same direction as that of incident light are taken as positive.
4. The distances measured against the direction of incident light are taken as negative.
5. The distances measured upward and perpendicular to the principal axis is taken as positive.
6. The distances measured downward and perpendicular to the principal axis is taken as negative.
46. Which of the following signifies the Magnification of lens?
- height of the image/ height of the object
- height of the image X height of the object
- height of the image + height of the object
- height of the image – height of the object
Explanation
Like spherical mirrors, we have magnification for spherical lenses. Spherical lenses produce magnification and it is defined as the ratio of the height of the image to the height of an object. Magnification is denoted by the letter ‘m’. If height of the object is h and height of the image is h´, the magnification produced by lens is,
m = height of the image /height of the object = h’ /h
47. If magnification is greater than 1, then we get a/an_____ image
- Same size
- Enlarged
- Diminished
- None
Explanation
If the magnification is greater than 1, then we get an enlarged image. On the other hand, if the magnification is less than 1, then we get a diminished image.
48. Which of the following statement about lens is correct?
- All lenses are made up of transparent materials
- Any optically transparent material will have a refractive index
- The lens formula relates the focal length of a lens with the distance of object and image.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
All lenses are made up of transparent materials. Any optically transparent material will have a refractive index. The lens formula relates the focal length of a lens with the distance of object and image. For a maker of any lens, knowledge of radii of curvature of the lens is required. This clearly indicates the need for an equation relating the radii of curvature of the lens, the refractive index of the given material of the lens and the required focal length of the lens.
49. The ability to converge or diverge these light rays depends on______ of the lens
- Principal axis
- Focal length
- Refractive index
- None
Explanation
It is clear that when a ray of light falls on a lens, the ability to converge or diverge these light rays depends on the focal length of the lens.
50. What is the formula to calculate the Power of a lens?
- 1 /f
- – f
- u – v
- f/x
Explanation
The ability of a lens to converge (convex lens) or diverge (concave lens) is called as its power. Hence, the power of a lens can be defined as the degree of convergence or divergence of light rays. Power of a lens is numerically defined as the reciprocal of its focal length.
P = 1 /f
51. What is the SI unit of Power of a lens?
- Dioptre
- m
- 1/m
- 1/D
Explanation
The SI unit of power of a lens is dioptre. It is represented by the symbol D. If focal length is expressed in ‘m’, then the power of lens is expressed in ‘D’. Thus, 1D is the power of a lens, whose focal length is 1 metre. 1D = 1m^-1
52. What is the power of concave lens?
- Positive
- Negative
- Neutral
- None
Explanation
By convention, the power of a convex lens is taken as positive whereas the power of a concave lens is taken, as negative. The lens formula and lens maker’s formula are applicable to only thin lenses. In the case of thick lenses, these formulae with little modifications are used.
53. What is the diameter of eye ball?
- 2.3 cm
- 2.3 m
- 12.3 cm
- 1.3 m
Explanation
The eye ball is approximately spherical in shape with a diameter of about 2.3 cm. It consists of a tough membrane called sclera, which protects the internal parts of the eye.
54. Which of the following refracts or bends the light on to the lens?
- Cornea
- Pupil
- Retina
- All the above
Explanation
Cornea is the thin and transparent layer on the front surface of the eyeball. It is the main refracting surface. When light enters through the cornea, it refracts or bends the light on to the lens.
55. _____ is the back surface of the eye
- Pupil
- Retina
- Ciliary muscles
- All the above
Explanation
Retina is the back surface of the eye. It is the most sensitive part of human eye, on which real and inverted image of objects is formed.
56. Which of the following are the possible colours of the Iris?
- Red
- Blue
- Green
- Brown
- 1, 3, 4
- 2, 3, 4
- 1, 2, 4
- All the above
Explanation
Iris is the coloured part of the eye. It may be blue, brown or green in colour. Every person has a unique colour, pattern and texture. Iris controls amount of light entering into the pupil like camera aperture.
57. Eye lens is fixed between_______
- Pupil
- Retina
- Ciliary muscles
- 1, 3
- 2, 3
- 3 alone
- 1, 2
Explanation
Eye lens is fixed between the ciliary muscles. It helps to change the focal length of the eye lens according to the position of the object.
58. Human eye is in nature?
- Concave
- Convex
- a or b
- None
Explanation
Pupil is the centre part of the Iris. It is the pathway for the light to retina. Eye Lens is the important part of human eye. It is convex in nature.
59. Which of the following statement is correct?
- The transparent layer cornea bends the light rays through pupil located at the centre part of the Iris.
- The adjusted light passes through the eye lens
- Retina passes the received real and inverted image to the brain through optical nerves
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
The transparent layer cornea bends the light rays through pupil located at the centre part of the Iris. The adjusted light passes through the eye lens. Eye lens is convex in nature. So, the light rays from the objects are converged and a real and inverted image is formed on retina. Then, retina passes the received real and inverted image to the brain through optical nerves. Finally, the brain senses it as erect image.
60. Assertion(A): The ability of the eye lens to focus nearby as well as the distant objects is called
power of accommodation of the eye
Reason(R): This is achieved by changing the focal length of the eye lens with the help of ciliary
muscles.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
The ability of the eye lens to focus nearby as well as the distant objects is called power of accommodation of the eye. This is achieved by changing the focal length of the eye lens with the help of ciliary muscles.
61. Which of the following statement is correct?
- Eye lens is made of a flexible, jelly-like material
- When we see distant objects, the ciliary muscle relaxes and makes the eye lens thinner
- when we look at a closer object, the focal length of the eye lens is decreased by the contraction of ciliary muscle
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Eye lens is made of a flexible, jelly-like material. By relaxing and contracting the ciliary muscle, the curvature and hence the focal length of the eye lens can be altered. When we see distant objects, the ciliary muscle relaxes and makes the eye lens thinner. This increases the focal length of the eye lens. Hence, the distant object can be clearly seen. On the other hand, when we look at a closer object, the focal length of the eye lens is decreased by the contraction of ciliary muscle. Thus, the image of the closer object is clearly formed on the retina.
62. What is the minimum time required by the eye to distinguish consecutive light pulses?
- 1 sec
- 0.1 sec
- 10 sec
- 1 msec
Explanation
If the time interval between two consecutive light pulses is less than 0.1 second, human eye cannot distinguish them separately. It is called persistence of vision.
63. What is the minimum distance required by human eye to see the objects distinctly without strain?
- 25 m
- 25 cm
- 15 cm
- 15 m
Explanation
The minimum distance required to see the objects distinctly without strain is called least distance of distinct vision. It is called as near point of eye. It is 25 cm for normal human eye.
64. What is the far point of the eye?
- 150 m
- 100 m
- 200 m
- Infinity
Explanation
The maximum distance up to which the eye can see objects clearly is called as far point of the eye. It is infinity for normal eye.
65. Which of the following statement is correct?
- A normal human eye can clearly see all the objects placed between 25cm and infinity
- But, for some people, the eye loses its power of accommodation.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
A normal human eye can clearly see all the objects placed between 25cm and infinity. But, for some people, the eye loses its power of accommodation. Th is could happen due to many reasons including ageing. Hence, their vision becomes defective.
66. Which of the following statement about myopia is correct?
- Myopia, also known as short sightedness
- It occurs due to the shortening of eye ball
- The focal length of eye lens is reduced or the distance between eye lens and retina increases
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Myopia, also known as short sightedness, occurs due to the lengthening of eye ball. With this defect, nearby objects can be seen clearly but distant objects cannot be seen clearly. Th e focal length of eye lens is reduced or the distance between eye lens and retina increases. Hence, the far point will not be infinity for such eyes and the far point has come closer.
67. Myopia can be corrected with_____
- Concave lens
- Convex lens
- Bi-focal lens
- None
Explanation
In the case of myopia, image of distant objects is/are formed before the retina. This defect can be corrected using a concave lens.
68. What is the formula to calculate the focal length of concave lens in the case of myopia?
- x y /x−y
- x /x−y
- y /x−y
- x−y / x y
Explanation
Let a person with myopia eye can see up to a distance x. Suppose that he wants to see all objects farther than this distance, i.e., up to infinity. Then the focal length of the required concave lens is f = –x. If the person can see up to a distance x and he wants to see up to a distance y, then, the focal length of the required concave lens is,
f = x y /x – y
69. Which of the following statement is correct about Hypermetropia?
- Hypermetropia, also known as long sightedness
- It occurs due to the shortening of eye ball
- Th e focal length of eye lens is increased or the distance between eye lens and retina decreases.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Hypermetropia, also known as long sightedness, occurs due to the shortening of eye ball. With this defect, distant objects can be seen clearly but nearby objects cannot be seen clearly. The focal length of eye lens is increased or the distance between eye lens and retina decreases. Hence, the near point will not be at 25cm for such eyes and the near point has moved farther. Due to this, the image of nearby objects is/are formed behind the retina. This defect can be corrected using a convex lens.
70. Which of the following statement is incorrect?
- Due to ageing, ciliary muscles become weak and the eye-lens become rigid (inflexible) and so the eye loses its power of accommodation.
- Presbyopia is also called as ‘old age hypermetropia’
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
Due to ageing, ciliary muscles become weak and the eye-lens become rigid (inflexible) and so the eye loses its power of accommodation. Because of this, an aged person cannot see the nearby objects clearly. So, it is also called as ‘old age hypermetropia’.
71. Presbyopia can be corrected with______ lens
- Concave
- Convex
- Bifocal
- It cannot be corrected
Explanation
Some persons may have both the defects of vision – myopia as well as hypermetropia. This can be corrected by bifocal lenses. In which, upper part consists of concave lens (to correct myopia) used for distant vision and the lower part consists of convex lens (to correct hypermetropia) used for reading purposes.
72. Astigmatism can be corrected by using________ lenses
- Concave
- Convex
- Bifocal
- Cylindrical
Explanation
In this defect, eye cannot see parallel and horizontal lines clearly. It may be inherited or acquired. It is due to the imperfect structure of eye lens because of the development of cataract on the lens, ulceration of cornea, injury to the refracting surfaces, etc. Astigmatism can be corrected by using cylindrical lenses (Torrid lenses).
73. Simple microscope has a ______lens of short focal length
- Concave
- Convex
- Bifocal
- Cylindrical
Explanation
Microscope works under the principle of angular magnification of lenses. It is classified as
1. Simple microscope
2. Compound microscope
Simple microscope has a convex lens of short focal length. It is held near the eye to get enlarged image of small objects.
74. Which of the following are the uses of Simple microscope?
- watch repairers and jewellers
- observe parts of flower, insects etc
- to observe finger prints in the field of forensic science.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Simple microscopes are used
a) by watch repairers and jewellers.
b) to read small letters clearly.
c) to observe parts of flower, insects etc.
d) to observe finger prints in the field of forensic science.
75. Which of the following statement is correct?
- Compound microscope is also used to see the tiny objects.
- It has better magnification power than simple microscope.
- Magnification power of microscopes can be increased by decreasing the focal length of the lens used
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Compound microscope is also used to see the tiny objects. It has better magnification power than simple microscope. Magnification power of microscopes can be increased by decreasing the focal length of the lens used. Due to constructional limitations, the focal length of the lens cannot be decreased beyond certain limit. This problem can be solved by using two separate biconvex lenses.
76. How many lenses are there in a compound microscope?
- 4
- 2
- 3
- 1
Explanation
A compound microscope consists of two convex lenses. The lens with the shorter focal length is placed near the object, and is called as ‘objective lens’ or ‘objective piece’. The lens with larger focal length and larger aperture placed near the observer’s eye is called as ‘eye lens’ or ‘eye piece’. Both the lenses are fixed in a narrow tube with adjustable provision.
77. How many times more magnification power than simple microscope than compound microscope
does have?
- 5 to 10
- 50 to 100
- 50 to 200
- 100 to 200
Explanation
Compound microscope has 50 to 200 times more magnification power than simple microscope. A travelling microscope is one of the best instruments for measuring very small length with high degree of accuracy at the order of 0.01mm. It works based on the principle of vernier. Its least count is 0.01 mm.
78. The first telescope was invented by_________
- Newton
- Galileo
- Jan Lippershey
- Einstein
Explanation
Telescope is an optical instrument to see the distant objects. The first telescope was invented by Jan Lippershey in 1608. Galileo made a telescope to observe distant stars. He got the idea, from a spectacle maker who one day observed that the distant weather cock appeared magnified through his lens system fitted in his shop.
79. Who invented a telescope which was fundamentally similar to the astronomical telescope?
- Newton
- Galileo
- Jan Lippershey
- Kepler
Explanation
Galileo observed the satellites of Jupiter and the rings of Saturn through his telescope. Kepler invented Telescope in 1611 which was fundamentally similar to the astronomical telescope.
80. According to optical property, telescopes are classified into___ types
- 3
- 4
- 2
- 5
Explanation
According to optical property, it is classified into two groups:
i) refracting telescope
ii) reflecting telescope
In refracting telescope lenses are used. Galilean telescope, Keplerian telescope, Achromatic refractors, Apochromatic refractors are some refracting telescopes.
In reflecting telescope parabolic mirrors are used Gregorian, Newtonian, Cassegrain telescope, Ritchey–Chrétien telescope are some Reflecting telescopes According to the things which are observed, Astronomical Telescope and Terrestrial Telescopes are the two major types of telescope
81. Which of the following statement is correct?
- The image in an astronomical telescope is real
- It is not suitable for viewing objects on the surface of the Earth
- The major difference between astronomical and terrestrial telescope is erecting the final image with respect to the object
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
The image in an astronomical telescope is inverted. So, it is not suitable for viewing objects on the surface of the Earth. Therefore, a terrestrial telescope is used. It provides an erect image. The major difference between astronomical and terrestrial telescope is erecting the final image with respect to the object.
82. A person with myopia can see objects placed at a distance of 4m. If he wants to see objects at a
distance of 20m, what should be the power of the concave lens he must wear?
- 0.2 D
- – 0.2 D
- 0.5 D
- – 0.5 D
Explanation

83. A beam of light passing through a diverging lens of focal length 0.3m appear to be focused at a
distance 0.2m behind the lens. Find the position of the object.
- −0.6 m
- 0.6 m
- 0.3 m
- – 0.3 m
Explanation
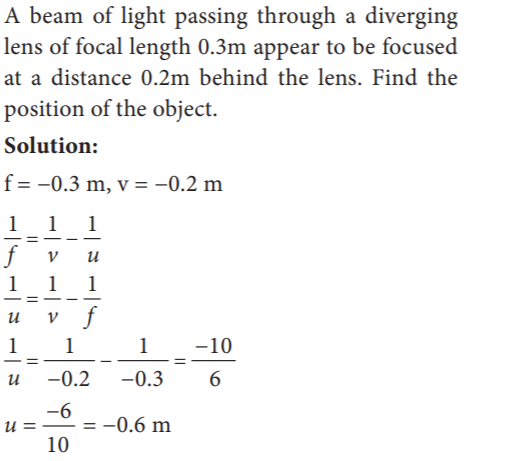
84. For a person with hypermetropia, the near point has moved to 1.5m. Calculate the focal length of
the correction lens in order to make his eyes normal.
- 0.3 m
- 0.6 m
- – 0.5 m
- – 0.6 m
Explanation

10th Science Lesson 3 Questions in English
1. Which of the following statement is correct?
- Sun is the primary source of thermal energy for all living organisms.
- Thermal energy is the effect, and temperature is the cause.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
Sun is the primary source of thermal energy for all living organisms. Thermal energy is the cause, and temperature is the effect. All living organisms need a particular temperature for their survival.
2. Assertion(A): Temperature can be defined as the property which determines whether a body is in
equilibrium or not with the surroundings
Reason(R): Temperature is defined as the degree of hotness of a body.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
Temperature is defined as the degree of hotness of a body. The temperature is higher for a hotter body than for a colder body. It is also be defined as the property which determines whether a body is in equilibrium or not with the surroundings.
3. What is the SI unit of temperature?
- degree Celsius
- degree Fahrenheit
- Kelvin
- All the above
Explanation
Temperature is the property, which determines the direction of flow of heat. It is a scalar quantity. The SI unit of temperature is kelvin (K). There are other commonly used units of temperature such as degree Celsius (°C) and degree Fahrenheit (°F).
4. The temperature measured in relation to absolute zero using the kelvin scale is known as_______
- Dynamic temperature
- Absolute temperature
- Normal temperature
- None
Explanation
The temperature measured in relation to absolute zero using the kelvin scale is known as absolute temperature. It is also known as the thermodynamic temperature.
5. A temperature difference of 1°C is equal to that of_________ K
- 273
- 1
- 10
- 1/273
Explanation
Each unit of the thermodynamic scale of temperature is defined as the fraction of 1/273.16th part of the thermodynamic temperature of the triple point of water. A temperature difference of 1°C is equal to that of 1K. Zero Kelvin is the absolute scale of temperature of the body.
6. 0K =
- –273°C
- 273°C
- 1°C
- 2C
Explanation
The relation between the different types of scale of temperature:
Celsius and Kelvin: K=C+ 273,
Fahrenheit and Kelvin: [K] = (F + 460) × 5 /9
0K = –273°C.
7. Two or more physical systems or bodies are said to be in thermal equilibrium if____
- There is no net flow of thermal energy between the systems
- There is net flow of thermal energy between the system
- There is partial flow of thermal energy between the system
- None
Explanation
Two or more physical systems or bodies are said to be in thermal equilibrium if there is no net flow of thermal energy between the systems. Heat energy always flows from one body to the other due to a temperature difference between them.
8. What will happen if two bodies at different temperatures are brought in contact with one other?
- There will be a transfer of heat energy from the hot body to the cold body
- There will be a transfer of heat energy from the cold body to the hot body
- Hot body will be hot and the cold body will be cold
- None
Explanation
There will be a transfer of heat energy from the hot body to the cold body until a thermal equilibrium is established between them. If two bodies are said to be in thermal equilibrium, then, they will be at the same temperature. When a cold body is placed in contact with a hot body, some thermal energy is transferred from the hot body to the cold body. As a result, there is some rise in the temperature of the cold body and decrease in the temperature of the hot body. This process will continue until these two bodies attain the same temperature.
9. Assertion(A): If you leave a cup of hot milk on a table for some time, the hotness of the milk
decreases after some time.
Reason(R): There is a flow of energy from the cup of milk to the environment.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
If you leave a cup of hot milk on a table for some time, what happens? The hotness of the milk decreases after some time. Similarly, if you keep a bottle of cold water on a table, the water becomes warmer after some time. What do you infer from these observations? In the case of hot milk, there is a flow of energy from the cup of milk to the environment. In the second case, the energy is transferred from the environment to the water bottle. This energy is termed as “thermal energy”.
10. Which of the following statement is correct?
- When a hot object is in contact with another cold object, a form of energy flows from the hot object to the cold object
- This energy is known as thermal energy
- Thermal energy is also known as ‘heat energy’ or simply ‘heat’.
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
When a hot object is in contact with another cold object, a form of energy flows from the hot object to the cold object, which is known as thermal energy. Thus, thermal energy is a form of energy which is transferred between any two bodies due to the difference in their temperatures. Thermal energy is also known as ‘heat energy’ or simply ‘heat’.
11. By which of the following process of transmission of heat takes place?
- Convection
- Conduction
- Radiation
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Heat energy is the agent, which produces the sensation of warmth and makes bodies hot. Process of transmission of heat may be done in any of the ways like conduction, convection or radiation.
12. What is the unit of heat energy?
- Joule
- Kelvin
- Degree centigrade
- Joule/ Kelvin
Explanation
The process in which heat energy flows from a body at a higher temperature to another object at lower temperature is known as heating. Heat is a scalar quantity. The SI unit of heat energy absorbed or evolved is joule (J).
13. Which of the following statement is incorrect?
- During the process of transferring heat energy, the body at lower temperature is heated while the body at higher temperature is cooled.
- When the thermal energy is transferred from one body to another, this results in the rise or lowering of the temperature of either of the bodies.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
During the process of transferring heat energy, the body at lower temperature is heated while the body at higher temperature is cooled. Thus, sometimes, this process of transfer of heat energy is termed as ‘cooling’. But, in most of the cases the term ‘heating’ is used instead of ‘cooling’. When the thermal energy is transferred from one body to another, this results in the rise or lowering of the temperature of either of the bodies.
14. Which of the following are the Characteristic features of heat energy transfer?
- Heat always flows from a system at higher temperature to a system at lower temperature.
- The mass of a system is altered when it is heated or cooled.
- Heat gained = Heat lost
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Characteristic features of heat energy transfer:
1. Heat always flows from a system at higher temperature to a system at lower temperature.
2. The mass of a system is not altered when it is heated or cooled.
3. For any exchange of heat, the heat gained by the cold system is equal to heat lost by the hot system. Heat gained = Heat lost.
15. Which of the following statement is incorrect?
- Though the SI unit of heat energy is joule, there are some other commonly used units such as Calorie
- One kilocalorie is defined as the amount of heat energy required to rise the temperature of 1 gram of water through 1°C
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
Though the SI unit of heat energy is joule, there are some other commonly used units:
Calorie: One calorie is defined as the amount of heat energy required to rise the temperature of 1 gram of water through 1°C.
Kilocalorie: One kilocalorie is defined as the amount of heat energy required to rise the temperature of 1 kilogram of water through 1°C.
16. Which of the following are the effects of heat energy in a substance?
- Temperature of the substance rises
- The substance will expand when heated
- The substance may change its state from solid to liquid or from liquid to gas
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
When a certain amount of heat energy is given to a substance, it will undergo one or more of the following changes:
- Temperature of the substance rises
- The substance may change its state from solid to liquid or from liquid to gas
- The substance will expand when heated.
17. Which of the following statement is correct?
- The rise in temperature is in proportion to the amount of heat energy supplied.
- It also depends on the nature of the substance alone
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
The rise in temperature is in proportion to the amount of heat energy supplied. It also depends on the nature and mass of the substance.
18. Assertion(A): The expansion of liquids (e. g. mercury) can be seen when a thermometer is placed
in warm water
Reason(R): When heat energy is supplied to a body, there can be an increase in the dimension of the object.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
When heat energy is supplied to a body, there can be an increase in the dimension of the object. The expansion of liquids (e. g. mercury) can be seen when a thermometer is placed in warm water.
19. Which of the following matter undergo thermal expansion?
- Liquid
- Gas
- Solid
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
The change in the dimension due to rise in temperature is called thermal expansion of the object. All forms of matter (solid, liquid and gas) undergo expansion on heating.
20. Assertion(A): For a given change in temperature, the extent of expansion is more in solids than
in liquids and gases.
Reason(R): When a solid is heated, the atoms gain energy and vibrate more vigorously.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is wrong and (R) is correct
Explanation
When a solid is heated, the atoms gain energy and vibrate more vigorously. This results in the expansion of the solid. For a given change in temperature, the extent of expansion is smaller in solids than in liquids and gases. This is due to the rigid nature of solids.
21. Which of the following are the types of expansion of solid?
- Ultrasonic expansion
- Linear expansion
- Superficial expansion
- Cubical expansion
- 1, 2, 4
- 2, 3, 4
- 1, 3, 4
- All the above
Explanation
The different types of expansion of solid are listed and explained below:
1. Linear expansion
2. Superficial expansion
3. Cubical expansion
22. The change in length of a body due to heating is called as__________
- Ultrasonic expansion
- Linear expansion
- Superficial expansion
- Cubical expansion
Explanation
When a body is heated or cooled, the length of the body changes due to change in its temperature. Then the expansion is said to be linear or longitudinal expansion.
23. What is the SI unit Coefficient of Linear expansion?
- K
- 1/K
- J
- J/K
Explanation
The SI unit of Coefficient of Linear expansion is K^-1. The value of coefficient of linear expansion is different for different materials. The ratio of increase in length of the body per degree rise in temperature to its unit length is called as the coefficient of linear expansion.
24. Which of the following is the equation relating the change in length and the change in
temperature of a body?
- ∆L/ Lo = αL ∆T
- ∆L * Lo = αL ∆T
- ∆L/ Lo = αL/ ∆T
- ∆L – Lo = αL ∆T
Explanation
The equation relating the change in length and the change in temperature of a body is given below:
∆L/ Lo = αL ∆T
∆L – Change in length (Final length – Original length)
Lo -Original length
∆T- Change in temperature (Final temperature – Initial temperature)
αL -Coefficient of linear expansion.
25. Which of the following statement about Superficial expansion is correct?
- It is also known as areal expansion
- If there is an increase in the area of a solid object due to heating, then the expansion is called superficial
- The SI unit of Coefficient of superficial expansion is K
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
If there is an increase in the area of a solid object due to heating, then the expansion is called superficial or areal expansion. The SI unit of Coefficient of superficial expansion is K^-1.
26. What does the coefficient of superficial expansion?
- The ratio of increase in area of the body per degree rise in temperature to its unit area
- The ratio of increase in length of the body per degree rise in temperature to its unit area
- The ratio of increase in volume of the body per degree rise in temperature to its unit area
- The ratio of increase in area of the body per degree rise in temperature to its unit length
Explanation
The ratio of increase in area of the body per degree rise in temperature to its unit area is called as coefficient of superficial expansion.
27. Which of the following statement correct about coefficient of superficial expansion??
- Coefficient of superficial expansion is different for different materials.
- Superficial expansion is determined in terms of coefficient of superficial expansion.
- ∆A/ Ao = αA ∆T
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Superficial expansion is determined in terms of coefficient of superficial expansion. Coefficient of superficial expansion is different for different materials.
∆A/ Ao = αA ∆T
∆A- Change in area (Final area – Initial area)
Ao -Original area
∆T- Change in temperature (Final temperature – Initial temperature)
αA-Coefficient of superficial expansion.
28. Which of the following statement is correct?
- If there is an increase in the volume of a solid body due to heating, then the expansion is called cubical or volumetric expansion
- The ratio of unit volume to its increase in volume of the body per degree rise in temperature is called as coefficient of cubical expansion.
- The unit of coefficient of cubical expansion is 1/K
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
If there is an increase in the volume of a solid body due to heating, then the expansion is called cubical or volumetric expansion. As in the cases of linear and areal expansion, cubical expansion is also expressed in terms of coefficient of cubical expansion. The ratio of increase in volume of the body per degree rise in temperature to its unit volume is called as coefficient of cubical expansion. This is also measured in K^–1.
29. The equation relating to the change in volume and the change in temperature is_______
- Vo/ ∆V = αV ∆T
- ∆V/ Vo = αV/ ∆T
- ∆V/ Vo = αV ∆T
- ∆V Vo = αV ∆T
Explanation
The equation relating to the change in volume and the change in temperature is given below:
∆V/ Vo = αV ∆T
∆V – Change in volume (Final volume – Initial volume)
Vo -Original volume
∆T- Change in temperature (Final temperature – Initial temperature)
αV -Coefficient of cubical expansion
Different materials possess different coefficient of cubical expansion.
30. Match the materials with their Coefficient of cubical expansion:
- Brass 1. 18.2 × 10^–5
- Glass 2. 2.5 × 10^–5
- Mercury 3. 7 × 10^–5
- Aluminium 4. 6 × 10^–5
- 3, 1, 2, 4
- 4, 2, 1, 3
- 4, 1, 2, 3
- 3, 2, 1, 4
Explanation
31. Which of the following substance has highest expansion when heated?
- Solid
- Liquid
- Gas
- Either a or b
Explanation
For a given rise in temperature, a liquid will have more expansion than a solid and a gaseous substance has the highest expansion when compared with the other two.
32. In which of the coefficient of cubical expansion of liquid is independent of temperature?
- Solid
- Liquid
- Gas
- None
Explanation
When heated, the atoms in a liquid or gas gain energy and are forced further apart. The coefficient of cubical expansion of liquid is independent of temperature whereas its value for gases depends on the temperature of gases.
33. Assertion(A): We can define real expansion and apparent expansion in liquid
Reason(R): The thermal energy supplied will be partly used in expanding the container and
partly used in expanding the liquid, this results in real expansion and apparent
expansion in liquid
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
When a liquid is heated, it is done by keeping the liquid in some container and supplying heat energy to the liquid through the container. The thermal energy supplied will be partly used in expanding the container and partly used in expanding the liquid. Thus, what we observe may not be the actual or real expansion of the liquid. Hence, for liquids, we can define real expansion and apparent expansion.
34. Which of the following statement is correct?
- If a liquid is heated directly without using any container, then the expansion that you observe is termed as real expansion of the liquid
- The SI unit of coefficient of real expansion is K^–1.
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
If a liquid is heated directly without using any container, then the expansion that you observe is termed as real expansion of the liquid. Coefficient of real expansion is defined as the ratio of the true rise in the volume of the liquid per degree rise in temperature to its unit volume. The SI unit of coefficient of real expansion is K^–1.
35. Assertion(A): The expansion of a liquid apparently observed without considering the expansion of the container is called the apparent expansion of the liquid
Reason(R): Heating a liquid without using a container is not possible. Thus, in practice, you can
heat any liquid by pouring it in a container.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
Heating a liquid without using a container is not possible. Thus, in practice, we can heat any liquid by pouring it in a container. Thus, what you observe is not the actual or real expansion of the liquid. The expansion of a liquid apparently observed without considering the expansion of the container is called the apparent expansion of the liquid. Coefficient of apparent expansion is defined as the ratio of the apparent rise in the volume of the liquid per degree rise in temperature to its unit volume. The SI unit of coefficient of apparent expansion is K^–1.
36. Let L1, L2 and L3 be level of liquid before heating, level of liquid appears to have reduced when heated and level of liquid due to expansion respectively. What is the real and Apparent expansion?
- L3 – L2, L3 – L1
- L3 – L1, L3 – L2
- L3 – L1, L2 – L1
- L3 – L2 – L1, L1 – L3
Explanation
The difference between the levels L1 and L3 is called as apparent expansion, and the difference between the levels L2 and L3 is called real expansion. The real expansion is always more than that of apparent expansion.
Real expansion = L3 – L2
Apparent expansion = L3 – L1
37. Which of the following is not a fundamental law of gases connecting pressure, volume,
temperature?
- Boyle’s Law
- Avogadro’s law
- Newton’s law
- Avogadro’s law
Explanation
The three fundamental laws which connect the relation between pressure, volume and temperature are as follows:
1) Boyle’s Law
2) Charles’s law
3) Avogadro’s law
38. According to Boyle’s law_____ is inversely proportional to pressure
- Temperature
- Volume of gas
- Mass of gas
- Area of gas
Explanation
When the temperature of a gas is kept constant, the volume of a fixed mass of gas is inversely proportional to its pressure.
P α 1/V
In other words, for an invariable mass of a perfect gas, at constant temperature, the product of its pressure and volume is a constant. (i.e.) PV = constant
39. Charles’s law was formulated by_______
- Jacques Charles
- Franki Charles
- Francis Charles
- Joseph Charles
Explanation
Charles’s law was formulated by a French scientist Jacques Charles. According to this law, When the pressure of gas is kept constant, the volume of a gas is directly proportional to the temperature of the gas.
V α T or V T = constant
40. The value of Avogadro number_______
- 6.023 × 1023 /mol
- 6.023 × 10^23 /mol
- 6.023 × 10^-23 /mol
- 6.023 × 10^26 /mol
Explanation
Avogadro’s law states that at constant pressure and temperature, the volume of a gas is directly proportional to number of atoms or molecules present in it.
(i.e.) V α n (or) V/ n = constant
Avogadro’s number (NA) is the total number of atoms per mole of the substance. It is equal to 6.023 × 10^23 /mol.
41. Which of the following statement is incorrect about Real Gases?
- If the molecules or atoms of a gases interact with each other with a definite amount of intermolecular or inter atomic force of attraction, then the gases are said to be real gases.
- At very high temperature or low pressure, real gas behaves as ideal gases
- 1 alone
- 2 alone
- 1, 2
- None
Explanation
If the molecules or atoms of a gases interact with each other with a definite amount of intermolecular or inter atomic force of attraction, then the gases are said to be real gases. At very high temperature or low pressure, real gas behaves as ideal gases because in this condition there is no interatomic or intermolecular force of attraction.
42. Are real gas and Ideal Gas are same?
- Yes
- No
- Yes, with some conditions
- None
Explanation
If the atoms or molecules of a gas do not interact with each other, then the gas is said to be an ideal gas or a perfect gas. In-case of real gases, they interact with each other with a definite amount of intermolecular or inter atomic force of attraction.
43. Assertion(A): In practice, no gas is ideal.
Reason(R): The molecules of any gas will have a certain amount of interaction among them.
- Both (A) and (R) are correct, but (R) does not explain (A)
- Both (A) and (R) are wrong
- Both (A) and (R) are correct and (R) explains (A)
- (A) is Correct and (R) is wrong
Explanation
Actually, in practice, no gas is ideal. The molecules of any gas will have a certain amount of interaction among them. But, these interactions are weaker when the pressure is low or the temperature is high because the interatomic or intermolecular forces of attraction are weak in ideal gas. Hence, a real gas at low pressure or high temperature can be termed as a perfect gas.
44. Ideal gases obey which of the following laws?
- Boyle’s law
- Charles’s law
- Avogadro’s law
- 1, 2
- 1, 3
- 2, 3
- All the above
Explanation
Ideal gases obey Boyle’s law, Charles’s law and Avogadro’s law. All these laws state the relationship between various properties of a gas such as pressure (P), volume (V), temperature (T) and number of atoms (n). In a given state of the gas, all these parameters will have a definite set of values. When there is a change in the state of the gas, any one or more of these parameters change its value. The above said laws relate these changes.
45. Which of the following is Ideal gas equation?
- PV = RT
- PT = RV
- PV = R/ T
- P/ V = R/ T
Explanation
Ideal gas equation is also called as equation of state because it gives the relation between the state variables and it is used to describe the state of any gas.
PV = RT
46. What is the value of Boltzmann constant?
- 1.38 × 10^–23 JK^ -1
- 1.38 × 10^–23 JK
- 1.38 × 10^–26 JK^–1
- 1.38 × 10^–26 JK^–1
Explanation
PV/ µNAT = constant
The value of the constant in the above equation is taken to be kB, which is called as Boltzmann constant (1.38 × 10^–23 JK^–1).
47. A container whose capacity is 70 ml is filled with a liquid up to 50 ml. Then, the liquid in the
container is heated. Initially, the level of the liquid falls from 50 ml to 48.5 ml. Then we heat
more, the level of the liquid rises to 51.2 ml. Find the apparent expansion.
- 1.2ml
- 2.7ml
- 1.5ml
- 1.7ml
Explanation

48. Keeping the temperature as constant, a gas is compressed four times of its initial pressure. The
volume of gas in the container changing from 20cc (V1 cc) to V2 cc. Find the final volume V2.
- 5 cm^3
- 15 cm^3
- 25 cm^3
- 50 cm^3
Explanation
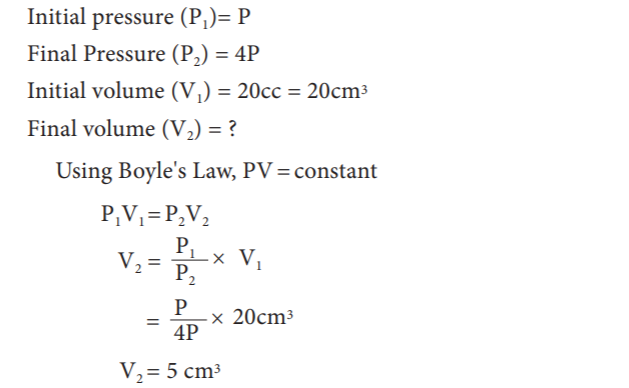
10th Science Lesson 4 Questions in English
4] Electricity
1. The motion of what through a conductor (e.g., copper wire) will constitute an electric current?
- Proton
- Electron
- Neutron
- All the above
Explanation
The motion of electric charges (electrons) through a conductor (e.g., copper wire) will constitute an electric current. This is similar to the flow of water through a channel or flow of air from a region of high pressure to a region of low pressure. In a similar manner, the electric current passes from the positive terminal (higher electric potential) of a battery to the negative terminal (lower electric potential) through a wire.
2. Which among the following is the symbol that represent the Electric current?
- I
- L
- W
- T
Explanation
Electric current is often termed as ‘current’ and it is represented by the symbol ‘I’. It is defined as the rate of flow of charges in a conductor. This means that the electric current represents the amount of charges flowing in any cross section of a conductor (say a metal wire) in unit time.
3. If a net charge ‘Q’ passes through any cross section of a conductor in time ‘t’, then the current flowing through the conductor is _____
- I = Q / T
- I = QT
- I = T / Q
- I = 1 / QT
Explanation
If a net charge ‘Q’ passes through any cross section of a conductor in time ‘t’, then the current flowing through the conductor is I = Q / T.
4. What is the SI unit of electric current?
- Newton
- Volt
- Ampere
- Joule
Explanation
The SI unit of electric current is ampere (A). The current flowing through a conductor is said to be one ampere, when a charge of one coulomb flows across any cross-section of a conductor, in one second. Hence, 1 ampere = 1 coulomb / 1 second.
5. A charge of 12 coulomb flows through a bulb in 5 second. What is the current through the bulb?
- 60 A
- 1.2 A
- 2.4 A
- 4.16 A
Explanation
Charge Q = 12 C, Time t = 5 s. Therefore,
Current I = Q /T = 12 / 5 = 2.4 A.
6. Which among the following component is used to fix the magnitude of the current through a circuit?
- Resistor
- A Diode
- Galvanometer
- Rheostat
Explanation
Resistor is used to fix the magnitude of the current through a circuit.
7. Which is used to select the magnitude of the current through a circuit?
- Light Emitting Diode
- A Diode
- Galvanometer
- Rheostat
Explanation
Variable resistor or Rheostat is used to select the magnitude of the current through a circuit.
8. Which among the following is used to detect the current and its direction?
- Ammeter
- Voltmeter
- Galvanometer
- Ground connection
Explanation
Galvanometer is used to detect the current and its direction.
9. Which among the following statement is correct
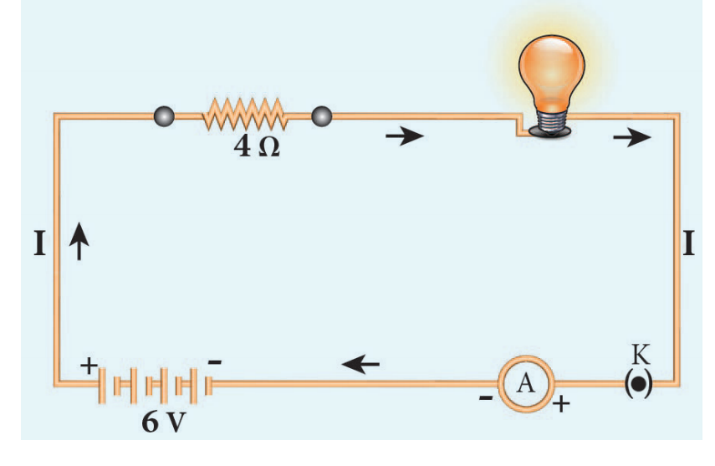
- An electric circuit is an open conducting loop (or) path, which has a network of electrical components through which electrons and protons are able to flow. This path is made using electrical wires so as to connect an electric appliance to a source of electric charges (battery).
- In this circuit, if the switch is ‘on’, the bulb glows. If it is switched off, the bulb does not glow. Therefore, the circuit must be closed in order that the current passes through it. The potential difference required for the flow of charges is provided by the battery. The electrons flow from the negative terminal to the positive terminal of the battery.
- By convention, the direction of current is taken as the direction of flow of positive charge (or) opposite to the direction of flow of electrons. Thus, electric current passes in the circuit from the positive terminal to the negative terminal.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
An electric circuit is a closed conducting loop (or) path, which has a network of electrical components through which electrons are able to flow. This path is made using electrical wires so as to connect an electric appliance to a source of electric charges (battery).
10. Which among the following component is used to measure the potential difference?
- Ammeter
- Voltmeter
- A diode
- Ground connection
Explanation
Voltmeter is used to measure the potential difference.
11. Which among the following components is wrongly matched with its unit
- Ammeter – Used to measure the difference in current and voltage
- A diode – It is used in electronic devices
- Ground connection – Used to provide protection to the electrical components. It also serves as a reference point to measure the electric potential
- Only 1
- Only 2
- Both 2 and 3
- None of the above
Explanation
12. Which among the following statement is correct
- In the conductor, the charges will flow from a point in it, which is at a lower electric potential to a point, which is at a higher electric potential.
- In the conductor, the charges will flow from a point in it, which is at a higher electric potential to a point, which is at a lower electric potential.
- There must be a difference in temperature between two points in a solid for the heat to flow in it. Similarly, a difference in electric potential is needed for the flow of electric charges in a conductor.
- Only 3
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
There must be a difference in temperature between two points in a solid for the heat to flow in it. Similarly, a difference in electric potential is needed for the flow of electric charges in a conductor. In the conductor, the charges will flow from a point in it, which is at a higher electric potential to a point, which is at a lower electric potential.
13. Which at a point is defined as the amount of work done in moving a unit positive charge from infinity to that point against the electric force?
- Electric resistance
- Electric conductance
- Electric manual
- Electric potential
Explanation
The electric potential at a point is defined as the amount of work done in moving a unit positive charge from infinity to that point against the electric force.
14. Which among the following statement is correct
- The electric potential difference between two points is defined as the amount of work done in moving a unit positive charge from one point to another point against the electric force.
- Suppose, you have moved a charge Q from a point A to another point B. Let ‘W’ be the work done to move the charge from A to B. Then, the potential difference between the points A and B is given by the following expression: Potential Difference (V) = work done (W) / charge (Q).
- Potential difference is inversely proportion to the difference in the electric potential of these two points. If VA and VB represent the electric potential at the points A and B respectively, then, the potential difference between the points A and B is given by: V = VA – VB (if VA is less than VB) V = VB – VA (if VB is less than VA).
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
Potential difference is also equal to the difference in the electric potential of these two points. If VA and VB represent the electric potential at the points A and B respectively, then, the potential difference between the points A and B is given by: V = VA – VB (if VA is more than VB) V = VB – VA (if VB is more than VA).
15. The SI unit of electric potential or potential difference is ___
- Joule
- Volt
- Newton
- Ohm
Explanation
The SI unit of electric potential or potential difference is volt (V).
16. Which among the following equation defines 1 Volt?
- 1 Joule / 1 Coulomb
- 1 Coulomb / 1 Joule
- 1 Joule × 1 Coulomb
- 1 Joule + 1 Coulomb
Explanation
The potential difference between two points is one volt, if one joule of work is done in moving one coulomb of charge from one point to another against the electric force.
1 Volt = 1 Joule / 1 Coulomb
17. Who among the following established the relation between the potential difference and current?
- Michael Faraday
- Gustav Kirchhoff
- Georg Simon Ohm
- Ernest Rutherford
Explanation
A German physicist, Georg Simon Ohm established the relation between the potential difference and current, which is known as Ohm’s Law. This relationship can be understood from the following activity.
18. Which among the following statement is correct
- According to Ohm’s law, at a constant temperature, the steady current ‘I’ flowing through a conductor is directly proportional to the potential difference ‘V’ between the two ends of the conductor. I is proportion to V. Hence, I / V = constant.
The value of this proportionality constant is found to be R. Therefore I = R V
- V = I R. Here, R is a constant for a given material (say Nichrome) at a given temperature and is known as the resistance of the material. Since, the potential difference V is proportional to the current I, the graph between V and I is a straight line for a conductor.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
According to Ohm’s law, at a constant temperature, the steady current ‘I’ flowing through a conductor is directly proportional to the potential difference ‘V’ between the two ends of the conductor. I is proportion to V. Hence, I / V = constant.
The value of this proportionality constant is found to be 1 / R. Therefore I = (1 / R) V.
19. The work done in moving a charge of 10 C across two points in a circuit is 100 J. What is the potential difference between the points?
- V = 0.1 volt
- V = 110 volt
- V = 1000 volt
- V = 10 volt
Explanation
Charge, Q = 10 C Work Done, W = 100 J
Potential Difference V = W / Q = 100 / 10
Therefore V = 10 volt.
20. Which can be defined as the ratio between the potential difference across the ends of the conductor and the current flowing through it?
- Inductance of a conductor
- Resistance of a conductor
- Capacity of conductor
- None of the above
Explanation
The resistance of a conductor can be defined as the ratio between the potential difference across the ends of the conductor and the current flowing through it.
21. Which among the following statement is correct
- In this Figure, a Nichrome wire was connected between X and Y. If you replace the Nichrome wire with a copper wire and conduct the same experiment, you will notice a same current for the same value of the potential difference across the wire. If you again replace the copper wire with an aluminium wire, you will same value again for the current passing through it.
- From equation V = I R, you have learnt that V/I must be equal to the resistance of the conductor used. The variations in the current for the same values of potential difference indicate that the resistance of different materials is different.
- Resistance of a material is its property to oppose the flow of charges and hence the passage of current through it. It is different for different materials. From Ohm’s Law, V / I = R.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
In this Figure, a Nichrome wire was connected between X and Y. If you replace the Nichrome wire with a copper wire and conduct the same experiment, you will notice a different current for the same value of the potential difference across the wire. If you again replace the copper wire with an aluminium wire, you will get another value for the current passing through it.
22. What is the SI unit of resistance?
- Newton
- Ohm
- Volt
- Coulomb
Explanation
The SI unit of resistance is ohm and it is represented by the symbol Ω.
23. Which among the following equation denotes Resistance of a conductor is said to be one ohm if a current of one ampere flows through it when a potential difference of one volt is maintained across its ends?
- 1 ohm = 1 volt / 1 ampere
- 1 ohm = 1 ampere / 1 volt
- 1 ohm = 1 ampere × 1 volt
- 1 ohm = 1 ampere + 1 volt
Explanation
Resistance of a conductor is said to be one ohm if a current of one ampere flows through it when a potential difference of one volt is maintained across its ends.
1 ohm = 1 volt / 1 ampere
24. Which among the following is the unit of Electric resistivity?
- Ohm
- Ohm metre
- Ohm-1
- Ohm metre-1
Explanation
The electrical resistivity of a material is defined as the resistance of a conductor of unit length and unit area of cross section. Its unit is ohm metre.
25. Which among the following statement is correct
- You can verify electric resistivity by doing an experiment that the resistance of any conductor ‘R’ is directly proportional to the length of the conductor ‘L’ and is inversely proportional to its area of cross section ‘A’. R . Therefore R = ρ (L/A)
- ρ (rho) is a constant, called as electrical resistivity or specific resistance of the material of the conductor. ρ = (RA) / L. If L = 1 m, A = 1 m2 then, from the above equation ρ = R. Electrical resistivity of a conductor is a measure of the resisting power of a specified material to the passage of an electric current. It is a constant for a given material.
- Only 1
- Only 2
- Both 1 and 2
- None
26. Calculate the resistance of a conductor through which a current of 2 A passes, when the potential difference between its ends is 30 V?
- 10 Ω
- 15 Ω
- 30 Ω
- 60 Ω
Explanation
Current through the conductor I = 2 A, Potential Difference V = 30 V
From Ohm’s Law: R = V / I
Therefore, R = 30 / 2 = 15 Ω.
27. Which is a conductor with highest resistivity equal to 1.5 × 10-6 Ω m?
- Aluminium
- Copper
- Silver
- Nichrome
Explanation
Nichrome is a conductor with highest resistivity equal to 1.5 × 10–6Ω m. Hence, it is used in making heating elements.
28. The reciprocal of electrical resistivity of a material is called _____
- Electrical Projective
- Electrical Conductivity
- Electrical Inductance
- All the above
Explanation
The reciprocal of electrical resistivity of a material is called its electrical conductivity. Conductance of a material is the property of a material to aid the flow of charges and hence, the passage of current in it. The conductance of a material is mathematically defined as the reciprocal of its resistance (R). Hence, the conductance ‘G’ of a conductor is given by G = 1 / R.
29. The unit of Conductance is ____
- Ohm
- Ohm metre
- Ohm-1
- Ohm metre-1
Explanation
The unit of conductance is Ohm-1. It is also represented as ‘mho’.
30. Which among the following is the Insulator?
- Copper
- Aluminium
- Rubber
- Nickle
Explanation
Electrical conductivity of a conductor is a measure of its ability to pass the current through it. Some materials are good conductors of electric current. Example: copper, aluminium, etc. While some other materials are non-conductors of electric current (insulators). Example: glass, wood, rubber, etc.
31. What is the unit of electrical conductivity?
- Ohm
- Ohm metre
- Ohm-1
- Ohm metre-1
Explanation
The unit of electrical conductivity is ohm-1 metre-1. It is also represented as mho metre-1. The conductivity is a constant for a given material.
32. Which among the following material materials resistivity is wrongly matched
- Copper – 1.62 × 10-8
- Nickel – 10.14 × 10–8
- Chromium – 12.9 × 10–8
- Only 1
- Only 2
- Both 1 and 3
- Both 2 and 3
Explanation
Copper – 1.62 × 10-8; Nickel – 6.84 × 10–8; Chromium – 12.9 × 10–8; Glass – 1010 to 1014; Rubber – 1013 to 1016.
33. The combination of resistors is known as ______
- System of resistors
- Network of resistors
- Bridge of resistors
- Gate of resistors
Explanation
The combination of resistors is known as ‘system of resistors’ or ‘grouping of resistors. Resistors can be connected in various combinations. The two basic methods of joining resistors together are: a) Resistors connected in series, and b) Resistors connected in parallel.
34. The resistance of a wire of length 10 m is 2 ohms. If the area of cross section of the wire is 2×10-7 m2, determine its (i) resistivity (ii) conductance and (iii) conductivity.
- 10-8 108m-1
- 10-8 108m-1
- 10-8 108m-1
- 10-8 108m-1
Explanation
Given: Length, L = 10 m, Resistance, R = 2 ohm and Area, A = 2 × 10–7 m2
Resistivity, = (2×2×10-7)/10 = 4 × 10-8 Ω m
Conductance, G = = 0.5 mho
Conductivity, 1/ (4 × 10-8) = 0.25 × 108 mho m–1.
35. Which among the following statement is correct
- A series circuit connects the components one after the other to form a ‘multiple loop’. A series circuit has two or more loop through which current can pass. If the circuit is interrupted at any point in the loop, no current can pass through the circuit and hence no electric appliances connected in the circuit will work.
- Series circuits are commonly used in devices such as flashlights. Thus, if resistors are connected end to end, so that the same current passes through each of them, then they are said to be connected in series.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
A series circuit connects the components one after the other to form a ‘single loop’. A series circuit has only one loop through which current can pass. If the circuit is interrupted at any point in the loop, no current can pass through the circuit and hence no electric appliances connected in the circuit will work.
36. Which among the following statement is correct
- Let, three resistances R1, R2 and R3 be connected in series (Figure 4.6). Let the current flowing through them be I. According to Joules Law, the potential differences V1, V2 and V3 across R1, R2 and R3 respectively, are given by V1 = IR1, V2 = IR2, V3 = IR3. The sum of the potential differences across the ends of each resistor is given by: V = V1 + V2 + V3 —-???? 1
- Using equations, we get V = I R1 + I R2 + I R3. The effective resistor is a single resistor, which can replace the resistors effectively, so as to allow the same current through the electric circuit. Let, the effective resistance of the series-combination of the resistors, be RS. Then, V = I RS. —???? 2
- Combining 1 and 2, I RS = I R1 + I R2 + I R3 i.e., RS = R1 + R2 + R3. Thus, you can understand that when a number of resistors are connected in series, their equivalent resistance or effective resistance is equal to the sum of the individual resistances. When ‘n’ resistors of equal resistance R are connected in series, the equivalent resistance is ‘n R’.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
Let, three resistances R1, R2 and R3 be connected in series (Figure 4.6). Let the current flowing through them be I. According to Ohm’s Law, the potential differences V1, V2 and V3 across R1, R2 and R3 respectively, are given by V1 = IR1, V2 = IR2, V3 = IR3. The sum of the potential differences across the ends of each resistor is given by: V = V1 + V2 + V3.
37. Which among the following statement is correct
- A parallel circuit has two or more loops through which current can pass. If the circuit is disconnected in one of the loops, the current can still pass through the other loop(s). The wiring in a house consists of parallel circuits. Consider that three resistors R1, R2 and R3 are connected across two common points A and B.
- The potential difference across each resistance is the same and equal to the potential difference between A and B. This is measured using the voltmeter. The current I arriving at A divides into three branches I1, I2 and I3 passing through R1, R2 and R3 respectively. According to the Ohm’s law, you have, I1 = V / R1, I2 = V / R2, I3 = V / R3. The total current through the circuit is given by I = I1 + I2 + I3 = V / R1 + V / R2 + V / R3. —????
- Let the effective resistance of the parallel combination of resistors be Rp. Then, I = V / Rp. combining 1 and 2 we have V / Rp = (V / R1)+(V / R2)+(V / R3) 1 / Rp = (1 / R1)+(1 / R2)+(1 / R3). Thus, when a number of resistors are connected in parallel, the sum of the reciprocals of the individual resistances is equal to the reciprocal of the effective or equivalent resistance. When ‘n’ resistors of equal resistances R are connected in parallel, the equivalent resistance is n / R. Hence, RP = n / R.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
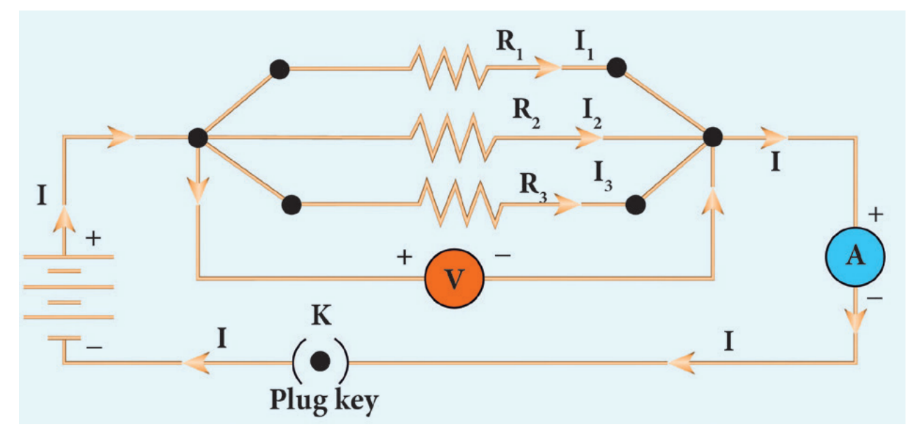
Let the effective resistance of the parallel combination of resistors be Rp. Then, I = V / Rp. combining 1 and 2 we have V / Rp = (V / R1)+(V / R2)+(V / R3) 1 / Rp = (1 / R1)+(1 / R2)+(1 / R3). Thus, when a number of resistors are connected in parallel, the sum of the reciprocals of the individual resistances is equal to the reciprocal of the effective or equivalent resistance. When ‘n’ resistors of equal resistances R are connected in parallel, the equivalent resistance is R / n. Hence, Rp = R / n.
38. Three resistors of resistances 5 ohms, 3 ohm and 2 ohms are connected in series with 10 V battery. Calculate their effective resistance and the current flowing through the circuit?
- RS = 15 Ω, I = 10A
- RS = 10 Ω, I = 1A
- RS = 15 Ω, I = 1A
- RS = 10 Ω, I = 15A
Explanation
R1 = 5 Ω, R2 = 3 Ω, R3 = 2 Ω, V = 10 V
Rs = R1 + R2 + R3, Rs = 5 + 3 + 2 = 10, hence
Rs = 10 Ω
The current, I = V / RS = 10 / 10 = 1 A.
39. Which among the following statement is correct
- If you consider the connection of a set of parallel resistors that are connected in series, you get a series – parallel circuit. Let R1 and R2 be connected in parallel to give an effective resistance of RP1. Similarly, let R3 and R4 be connected in parallel to give an effective resistance of RP2. Then, both of these parallel segments are connected in series.
1 / Rp1 = (1 / R1)+(1 / R2); 1 / Rp2 = (1 / R3)+(1 / R4) Hence, RTotal = RP1 + RP2
- If you consider a connection of a set of series resistors connected in a parallel circuit, you get a parallel-series circuit. Let R1 and R2 be connected in series to give an effective resistance of RS1. Similarly, let R3 and R4 be connected in series to give an effective resistance of RS2. Then, both of these serial segments are connected in parallel.
RS1 = R1 + R2, RS2 = R3 + R4. Hence, 1 / RTotal = (1 / RS1)+(1 / RS2)
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation

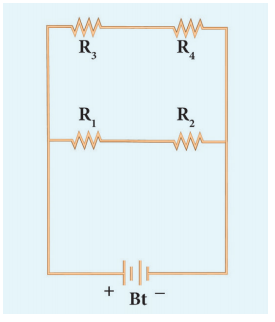
Series-parallel combination of resistors Parallel-series combination of resistors
40. Which among the following is not the property of series circuit?
- Equivalent resistance is more than the highest resistance
- Current is more as effective resistance is more
- If one appliance is disconnected, others also do not work
- None of the above
Explanation
Current is less as effective resistance is more.
41. Which among the following is not the property of parallel circuit?
- Equivalent resistance is less than the lowest resistance
- Current is more as effective resistance is less
- If one appliance is disconnected, others will work independently
- None of the above
42. Which among the following statement is correct
- Generally, a source of electrical energy can develop a potential difference across a capacitor, which is connected to that source. This potential difference constitutes a current through the capacitor. For continuous drawing of current, the source has to continuously spend its energy.
- A part of the energy from the source can be converted into useful work and the rest will be converted into heat energy. Thus, the passage of electric current through a wire, results in the production of heat. This phenomenon is called heating effect of current. This heating effect of current is used in devices like electric heater, electric iron, etc.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Generally, a source of electrical energy can develop a potential difference across a resistor, which is connected to that source. This potential difference constitutes a current through the resistor. For continuous drawing of current, the source has to continuously spend its energy.
43. Let ‘I’ be the current flowing through a resistor of resistance ‘R’, and ‘V’ be the potential difference across the resistor. The charge flowing through the circuit for a time interval ‘t’ is ‘Q’. The work done in moving the charge Q across the ends of the resistor with a potential difference of V is VQ. What is heat produced in resistor?
- H = R / I t
- H = I2 R t
- H = I2 / R t
- H = I R t
Explanation
This energy spent by the source gets dissipated in the resistor as heat. Thus, the heat produced in the resistor is: H = W = VQ
You know that the relation between the charge and current is Q = I t. Using this, you get H = V I t
From Ohm’s Law, V = I R. Hence, you have H = I2 R t.
This is known as Joule’s law of heating.
44. Which among the following does not states the joules law of heating that the heat produced in any resistor is
- directly proportional to the square of the current passing through the resistor
- directly proportional to the resistance of the resistor.
- directly proportional to the time for which the current is passing through the resistor.
- directly proportional to the series of resistance connected.
Explanation
Joule’s law of heating states that the heat produced in any resistor is: • directly proportional to the square of the current passing through the resistor. • directly proportional to the resistance of the resistor. • directly proportional to the time for which the current is passing through the resistor.
45. Which among the following is not the property of Nichrome which is used as the heating element?
- High resistivity
- High melting point
- Easily oxidized
- None of the above
Explanation
The heating effect of electric current is used in many home appliances such as electric iron, electric toaster, electric oven, electric heater, geyser, etc. In these appliances Nichrome, which is an alloy of Nickel and Chromium is used as the heating element. Because: (i) it has high resistivity, (ii) it has a high melting point, (iii) it is not easily oxidized.
46. How fuse wire is connected in an electric circuit, which when a large current pass through the circuit, the fuse wire melts due to Joule’s heating effect and hence the circuit gets disconnected?
- Parallel
- Series
- Series- parallel combination
- Series-parallel combination
Explanation
The fuse wire is connected in series, in an electric circuit. When a large current pass through the circuit, the fuse wire melts due to Joule’s heating effect and hence the circuit gets disconnected. Therefore, the circuit and the electric appliances are saved from any damage. The fuse wire is made up of a material whose melting point is relatively low.
47. In electric bulbs, a small wire made up of a material whose melting point is very high is used, known as ______
- Chock
- Insulation
- Stem
- Filament
Explanation
In electric bulbs, a small wire is used, known as filament. The filament is made up of a material whose melting point is very high. When current passes through this wire, heat is produced in the filament. When the filament is heated, it glows and gives out light. Tungsten is the commonly used material to make the filament in bulbs.
48. An electric heater of resistance 5 Ω is connected to an electric source. If a current of 6 A flows through the heater, then find the amount of heat produced in 5 minutes.
- H = 18000 J
- H = 36000 J
- H = 54000 J
- H = 72000 J
Explanation
Given resistance R = 5 Ω, Current I = 6 A, Time t = 5 minutes = 5 × 60 s = 300 s
Amount of heat produced, H = I2 Rt, H = 62 × 5 × 300. Hence, H = 54000 J
49. In general, what is defined as the rate of doing work or rate of spending energy?
- Power
- Static
- Modem
- Wire
Explanation
In general, power is defined as the rate of doing work or rate of spending energy. Similarly, the electric power is defined as the rate of consumption of electrical energy. It represents the rate at which the electrical energy is converted into some other form of energy.
50. Suppose a current ‘I’ flows through a conductor of resistance ‘R’ for a time ‘t’, then the potential difference across the two ends of the conductor is ‘V’. The work done ‘W’ to move the charge across the ends of the conductor is given by what equation?
- W = V I t
- W = V I / t
- W = V / I t
- W = I t / V
Explanation
The work done ‘W’ to move the charge across the ends of the conductor is given by the equation
W = V I t, Power P = work / Time = (V I t)/t = V I
P = V I. Thus, the electric power is the product of the electric current and the potential difference due to which the current passes in a circuit.
51. The SI unit of electric power is _____
- Ohm
- Newton
- Watt
- Coulomb
Explanation
The SI unit of electric power is watt. When a current of 1 ampere passes across the ends of a conductor, which is at a potential difference of 1 volt, then the electric power is
P = 1 volt × 1 ampere = 1 watt. Thus, one watt is the power consumed when an electric device is operated at a potential difference of one volt and it carries a current of one ampere. A larger unit of power, which is more commonly used is kilowatt.
52. Which is a unit in the foot-pound-second (fps) or English system, sometimes used to express the electric power?
- Coulomb
- Horse power
- Newton metre
- None of the above
Explanation
The horse power (hp) is a unit in the foot-pound-second (fps) or English system, sometimes used to express the electric power.
53. One horse power is equal to how many watts?
- 451 watts
- 572 watts
- 746 watts
- 824 watts
Explanation
One horse power is equal to 746 watts
54. Which among the following statement is correct
- Electricity is consumed both in houses and industries. Consumption of electricity is based on two factors: (i) Amount of electric power and (ii) Duration of usage. Electrical energy consumed is taken as the product of electric power and time of usage.
- Consumption of electrical energy is measured and expressed in watt hour, though its SI unit is watt second. In practice, a larger unit of electrical energy is needed. This larger unit is kilowatt hour (kWh).
- One kilowatt hour is otherwise known as one unit of electrical energy. One kilowatt hour means that an electric power of 100 watt has been utilized for an hour. Hence,
1 kWh = 100 watts hour = 100 × (60 × 60) watt second = 3.6 × 105 J.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
One kilowatt hour is otherwise known as one unit of electrical energy. One kilowatt hour means that an electric power of 1000 watt has been utilized for an hour. Hence,
1 kWh = 1000 watts hour = 1000 × (60 × 60) watt second = 3.6 × 106 J.
55. Which among the following statement is correct
- The electricity produced in power stations is distributed to all the domestic and industrial consumers through overhead and underground cables. In our homes, electricity is distributed through the domestic electric circuits wired by the electricians.
- The first stage of the domestic circuit is to bring the power supply to the main-box from a distribution panel, such as a transformer. The important components of the main-box are: (i) a fuse box and (ii) a meter. The meter is used to record the consumption of electrical energy. The fuse box contains either a fuse wire or a miniature circuit breaker (MCB).
- The function of the fuse wire or a MCB is to protect the house hold electrical appliances from overloading due to excess current. An MCB is a switching device, which can be activated automatically as well as manually. It has a spring attached to the switch, which is attracted by an electromagnet when an excess current pass through the circuit. Hence, the circuit is broken and the protection of the appliance is ensured.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
56. In India, domestic circuits are supplied with an alternating current of potential what?
- 110/120 V
- 220/230 V
- 310/320 V
- 420/430 V
Explanation
In India, domestic circuits are supplied with an alternating current of potential 220/230V and frequency 50 Hz.
57. In countries like USA and UK, domestic circuits are supplied with an alternating current of potential 110/120 V and frequency of what?
- 50 Hz
- 60 Hz
- 70 Hz
- 80 Hz
Explanation
In countries like USA and UK, domestic circuits are supplied with an alternating current of potential 110/120 V and frequency 60 Hz.
58. The electricity is brought to houses by how many insulated wires?
- Two
- Three
- Five
- Six
Explanation
The electricity is brought to houses by two insulated wires.
59. Out of the two Insulated wires brought to house, one wire has a red insulation and is called ____
- Flaw wire
- Neutral wire
- Trip wire
- Live wire
Explanation
Out of the two Insulated wires brought to house, one wire has a red insulation and is called the ‘live wire’. The other wire has a black insulation and is called the ‘neutral wire’.
60. Which among the following statement is correct
- The electricity supplied to your house is actually an direct current having an electric potential of 220 V. Both, the live wire and the neutral wire enter into a box where the main fuse is connected with the live wire.
- After the electricity meter, these wires enter into the main switch, which is used to discontinue the electricity supply whenever required. After the main switch, these wires are connected to live wires of two separate circuits. Out of these two circuits, one circuit is of a 5 A rating, which is used to run the electric appliances with a lower power rating, such as tube lights, bulbs and fans.
- The other circuit is of a 15 A rating, which is used to run electric appliances with a high-power rating, such as air-conditioners, refrigerators, electric iron and heaters. It should be noted that all the circuits in a house are connected in parallel, so that the disconnection of one circuit does not affect the other circuit.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
The electricity supplied to your house is actually an alternating current having an electric potential of 220 V. Both, the live wire and the neutral wire enter into a box where the main fuse is connected with the live wire.
61. In which among the following circuit, each electric appliance gets an equal voltage?
- Series connection
- Parallel connection
- Series-parallel combination
- Parallel-series combination
Explanation
One more advantage of the parallel connection of circuits is that each electric appliance gets an equal voltage.
62. The fuse wire or MCB will not disconnect the circuit in the event of what?
- Short circuit
- Overloading
- Weathering
- None of the above
Explanation
The fuse wire or MCB will disconnect the circuit in the event of an overloading and short circuiting.
63. When a live wire comes in contact with a neutral wire, it causes what?
- Over loading
- Short circuit
- Transcription
- All the above
Explanation
When a live wire comes in contact with a neutral wire, it causes a ‘short circuit’. This happens when the insulation of the wires gets damaged due to temperature changes or some external force. Due to a short circuit, the effective resistance in the circuit becomes very small, which leads to the flow of a large current through the wires.
64. What happens when a large number of appliances are connected in series to the same source of electric power?
- Over loading
- Transcription
- Power fluctuation
- Shortage
Explanation
Over loading happens when a large number of appliances are connected in series to the same source of electric power. This leads to a flow of excess current in the electric circuit. When the amount of current passing through a wire exceeds the maximum permissible limit, the wires get heated to such an extent that a fire may be caused.
65. In domestic circuit which wire is used as third wire having a green insulation which is usually connected to the body of the metallic electric?
- Tear wire
- Live wire
- Sub wire
- Earth wire
Explanation
In domestic circuits, a third wire called the earth wire having a green insulation is usually connected to the body of the metallic electric appliance. The other end of the earth wire is connected to a metal tube or a metal electrode, which is buried into the Earth. This wire provides a low resistance path to the electric current. The earth wire sends the current from the body of the appliance to the Earth, whenever a live wire accidentally touches the body of the metallic electric appliance which saves us from electric shock.
66. Two bulbs are having the ratings as 60 W, 220 V and 40 W, 220 V respectively. Which one has a greater resistance?
- The bulb with 60 W, 220 V rating has a greater resistance
- The bulb with 40 W, 220 V rating has a greater resistance
- Both the bulb has the same resistance
- None of the above
Explanation
Electric power P = V2/ R
For the same value of V, R is inversely proportional to P.
Therefore, lesser the power, greater the resistance
Hence, the bulb with 40 W, 220 V rating has a greater resistance.
67. With the help of which chemical compounds, the manufacturer can produce LED bulbs that radiates red, green, yellow and orange colours?
- Cadmium Selenide
- Silicon Carbide
- Gallium Arsenide
- Aluminium Nitride
Explanation
An LED bulb is a semiconductor device that emits visible light when an electric current pass through it. The colour of the emitted light will depend on the type of materials used. With the help of the chemical compounds like Gallium Arsenide and Gallium Phosphide, the manufacturer can produce LED bulbs that radiates red, green, yellow and orange colours.
68. Calculate the current and the resistance of a 100 W, 200 V electric bulb in an electric circuit?
- I = 2 A; R = 100 Ω
- I = 1.5 A; R = 300 Ω
- I = 1 A; R = 200 Ω
- I = 0.5 A; R = 400 Ω
Explanation
Power P = 100 W and Voltage V = 200 V Power P = V I
So, Current, I = P / V = 100 / 200 = 0.5 A
Resistance, R = V / I = 200 / 0.5 = 400 Ω
69. Which among the following is incorrect about LED bulb?
- As there is one large filament, there is less loss of energy in the form of heat. It is cooler than the incandescent bulb. It is not harmful to the environment. A wide range of colours is possible here.
- In comparison with the fluorescent light, the LED bulbs have significantly low power requirement. It is cost-efficient and energy efficient. Mercury and other toxic materials are not required.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
As there is no filament, there is no loss of energy in the form of heat. It is cooler than the incandescent bulb. It is not harmful to the environment. A wide range of colours is possible here.
70. In the circuit diagram given below, three resistors R1, R2 and R3 of 5 Ω, 10 Ω and 20 Ω respectively are connected as shown. Calculate:
i) Current through each resistor ii) Total current in the circuit iii) Total resistance in the circuit
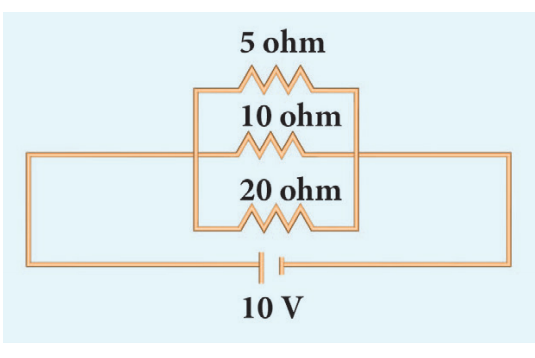
- R1 = 2A, R2 = 1A, R3 = 0.5A; I = 3.5A; RP = 2.857 Ω
- R1 = 0.5A, R2 = 1A, R3 = 2A; I = 3.5A; RP = 2.857 Ω
- R1 = 1.5A, R2 = 2A, R3 = 3A; I = 6.5A; RP = 2.857 Ω
- R1 = 2A, R2 = 3A, R3 = 1A; I = 6A; RP = 2.857 Ω
Explanation
i) Since the resistors are connected in parallel, the potential difference across each resistor is same (i.e., V=10V) Therefore, the current through R1 is I1 = V / R1 = 10 / 5 = 2A;
R2 = 10 / 10 = 1A;
R3 = 10 / 20 = 0.5A.
ii) Total current in the circuit, I = I1 + I2 + I3 = = 2 + 1 + 0.5 = 3.5 A
iii) Total resistance in the circuit 1 / RP = (1/R1) + (1/R2) + (1/R3) = (1/5) + (1/10) + (1/20)
= (4+2+1)/20 = 7/20. Hence, Rp = 20/7 = 2.857 Ω.
71. LEDs emitting which colour light are used in monochrome TV?
- Blue
- Red
- Black
- White
Explanation
LED Television is one of the most important applications of Light Emitting Diodes. An LED TV is actually an LCD TV (Liquid Crystal Display) with LED display. An LED display uses LEDs for backlight and an array of LEDs act as pixels. LEDs emitting white light are used in monochrome (black and white) TV.
72. Which among the following colour LEDs are not used in colour television?
- Red
- Green
- Violet
- Blue
Explanation
Red, Green and Blue (RGB) LEDs are used in colour television
73. Which is the display device used to give an output in the form of numbers or text?
- Seven Segment Display
- Eight Segment Display
- Nine Segment Display
- Ten Segment Display
Explanation
A ‘Seven Segment Display’ is the display device used to give an output in the form of numbers or text. It is used in digital meters, digital clocks, micro wave ovens, etc. It consists of 7 segments of LEDs in the form of the digit 8. These seven LEDs are named as a, b, c, d, e, f and g.
74. The first LED television screen was developed by whom in 1977?
- James P Mitchell
- Ada Lovelace
- William A Dubilier
- Reginald Fessenden
Explanation
The first LED television screen was developed by James P. Mitchell in 1977. It was a monochromatic display. But, after about three decades, in 2009, SONY introduced the first commercial LED Television.
75. Three resistors of 1 Ω, 2 Ω and 4 Ω are connected in parallel in a circuit. If a 1 Ω resistor draws a current of 1 A, find the current through the other two resistors?
- I2 = 0.25 A, I3 = 0.5 A
- I2 = 0.5 A, I3 = 0.25 A
- I2 = 1 A, I3 = 1.5 A
- I2 = 0.25 A, I3 = 0.1 A
Explanation
R1 = 1 Ω, R2 = 2 Ω, R3 = 4 Ω Current I1 = 1 A
The potential difference across the 1 Ω resistor = I1 R1 = 1 × 1 = 1 V
Since, the resistors are connected in parallel in the circuit, the same potential difference will exist across the other resistors also.
So, the current in the 2 Ω resistor, I2 = V / R2 = 1 / 2 = 0.5 A
Similarly, the current in the 4 Ω resistor, I3 = V / R3 = 1 / 4 = 0.25 A.
10th Science Lesson 5 Questions in English
5] Acoustics
1. Which is a branch of physics that deals with production, transmission, reception, control, and effects of sound?
- Optics
- Classis Mechanics
- Quantum Statistic
- Acoustics
Explanation
Acoustics is a branch of physics that deals with production, transmission, reception, control, and effects of sound.
2. By touching a ringing bell or a musical instrument while it is producing music, you can conclude that sound is produced by what?
- Air
- Vibrations
- Vacuum
- None of the above
Explanation
By touching a ringing bell or a musical instrument while it is producing music, you can conclude that sound is produced by vibrations. The vibrating bodies produce energy in the form of waves, which are nothing but sound waves.
3. Which among the following is not the medium for sound propagation?
- Air
- Water
- Vacuum
- Steel
Explanation
As the Moon does not have air, you will not be able to hear any sound produced by your friend. Hence, you understand that the sound produced due to the vibration of different bodies needs a material medium like air, water, steel, etc, for its propagation. Hence, sound can propagate through a gaseous medium or a liquid medium or a solid medium.
4. Which among the following statement is correct
- Sound waves are latitudinal waves that can travel through any medium (solids, liquids, gases) with the same speed irrespective of the properties of the medium. As sound travels through a medium, the particles of the medium vibrate along the direction of propagation of the wave.
- This displacement involves the longitudinal displacements of the individual molecules from their mean positions. This results in a series of high- and low-pressure regions called compressions and rarefactions.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Sound waves are longitudinal waves that can travel through any medium (solids, liquids, gases) with a speed that depends on the properties of the medium. As sound travels through a medium, the particles of the medium vibrate along the direction of propagation of the wave.
5. Which among the following wave are sound waves with a frequency below 20 Hz that cannot be heard by the human ear?
- Audible waves
- Ultrasonic waves
- Infrasonic waves
- All the above
Explanation
Infrasonic waves are sound waves with a frequency below 20 Hz that cannot be heard by the human ear.
6. Which among the following are sound waves with a frequency greater than 20 kHz, Human ear cannot detect these waves, but certain creatures like mosquito, dogs, bats, dolphins can detect these waves?
- Audible waves
- Ultrasonic waves
- Infrasonic waves
- All the above
Explanation
Ultrasonic waves are sound waves with a frequency greater than 20 kHz, Human ear cannot detect these waves, but certain creatures like mosquito, dogs, bats, dolphins can detect these waves. e.g., waves produced by bats.
7. What was the frequency range of Audible waves?
- 20 Hz to 200 Hz
- 20 Hz to 1000 Hz
- 20 Hz to 10000 Hz
- 20 Hz to 20000 Hz
Explanation
Audible waves are sound waves with a frequency ranging between 20 Hz and 20,000 Hz. These are generated by vibrating bodies such as vocal cords, stretched strings etc.
8. Which among the following is not the Infrasonic waves?
- Waves produced during earth quake
- Ocean waves
- Sound produced by whales
- Waves produced by bats
Explanation
Examples of Infrasonic waves are waves produced during earth quake, ocean waves, sound produced by whales, etc.
9. Which among the following is not the property of sound?
- Medium is required for the propagation
- Sound waves are longitudinal
- Wavelength ranges from 4 × 10–7 m to 7 × 10–7 m
- Sound waves travel in air with a speed of about 340 m s–1 at NTP
Explanation
Wavelength ranges from 1.65 cm to 1.65 m.
10. Which among the following is the SI unit of velocity?
- m s
- m s-1
- m s-2
- m-1 s-1
Explanation
The SI unit of velocity is ms-1
11. When you talk about the velocity associated with any wave, there are how many velocities?
- Two
- Three
- Five
- Six
Explanation
When you talk about the velocity associated with any wave, there are two velocities, namely particle velocity and wave velocity.
12. Which among the following statement is correct
- The velocity with which the particles of the medium vibrate in order to transfer the energy in the form of a wave is called particle velocity.
- The velocity with which the wave travels through the medium is called wave velocity. In other words, the distance travelled by a sound wave in unit time is called the velocity of a sound wave.
- Only 1
- Only 2
- Both 1 and 2
- None
13. Which among the following represent the expression for velocity?
- V = λ / T
- V = T / λ
- V = λ T
- V = λ T-2
Explanation
If the distance travelled by one wave is taken as one wavelength (λ) and, the time taken for this propagation is one time period (T), then, the expression for velocity can be written as ∴ V = λ / T.
Velocity = Distance / Time taken
Therefore, velocity can be defined as the distance travelled per second by a sound wave. Since, Frequency (n) =1/T, equation can be written as V = n λ
14. Velocity of sound is maximum in which among the following medium?
- Solid
- Liquid
- Gases
- All the above
Explanation
Velocity of a sound wave is maximum in solids because they are more elastic in nature than liquids and gases. Since, gases are least elastic in nature, the velocity of sound is the least in a gaseous medium. So, VS > VL > VG.
15. Which among the following statement is incorrect
- The speed of sound is directly proportional to the square root of the elastic modulus
- The speed of sound is inversely proportional to the square root of the density
- The speed of sound is directly proportion to the square root of the density
- None of the above
Explanation
In the case of solids, the elastic properties and the density of the solids affect the velocity of sound waves. Elastic property of solids is characterized by their elastic moduli. The speed of sound is directly proportional to the square root of the elastic modulus and inversely proportional to the square root of the density. Thus, the velocity of sound in solids decreases as the density increases whereas the velocity of sound increases when the elasticity of the material increases.
16. Which among the following statement is correct
- The velocity of sound in a gas is inversely proportional to the square root of the density of the gas. Hence, the velocity decreases as the density of the gas increases. V α (√1/d)
- The velocity of sound in a gas is inversely proportional to the square root of its temperature. The velocity of sound in a gas increases with the decrease in temperature. v ∝ √1/T. Velocity at temperature T is given by the following equation: vT = (vo + 0.61 T) m s–1.
- When humidity increases, the speed of sound increases. That is why you can hear sound from long distances clearly during rainy seasons.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
The velocity of sound in a gas is directly proportional to the square root of its temperature. The velocity of sound in a gas increases with the increase in temperature. v ∝ √T. Velocity at temperature T is given by the following equation: vT = (vo + 0.61 T) m s–1.
Here, vo is the velocity of sound in the gas at 0° C. For air, vo = 331 m s–1. Hence, the velocity of sound changes by 0.61 m s–1 when the temperature changes by one degree Celsius.
17. In which among the following medium sound travels at high speed?
- Copper
- Iron
- Aluminium
- All the above
Explanation
Solid medium ???? Copper = 5010 m s-1, Iron = 5950 m s-1 and Aluminium = 6420 m s-1.
18. In which among the following medium sound travels at high speed?
- Sea water
- Kerosene
- Water
- Air (at 0o C)
Explanation
Liquid medium???? Kerosene = 1324 m s-1, Water = 1493 m s-1, Sea water = 1533 m s-1, Air (at 0o C) = 331 m s-1 and Air (at 20o C) = 343 m s-1.
19. At what temperature will the velocity of sound in air be double the velocity of sound in air at 0o C?
- T = 628o C
- T = 736o C
- T = 783o C
- T = 819o C
Explanation
Let To C be the required temperature. Let v1 and v2 be the velocity of sound at temperatures T1 K and T2 K respectively. T1 = 273K (0o C) and T2 = (To C + 273) K.
v2/ v1 = (√T2 /T1) = √ ((273 + T) /273) = 2
Here, it is given that, v2 / v1 = 2.
So, (273 + T) / 273 = 4,
T = (273 × 4) – 273 = 819o C.
20. When sound waves travel in a given medium and strike the surface of another medium, they can be bounced back into the first medium. This phenomenon is known as _____
- Reflection
- Convention
- Echo
- Suspension
Explanation
When sound waves travel in a given medium and strike the surface of another medium, they can be bounced back into the first medium. This phenomenon is known as reflection. In simple the reflection and refraction of sound is actually similar to the reflection of light. Thus, the bouncing of sound waves from the interface between two media is termed as the reflection of sound.
21. The sound waves that strike the interface are termed as ______
- Transmitted wave
- Incident wave
- Dimension wave
- Narrow wave
Explanation
The waves that strike the interface are termed as the incident wave and the waves that bounce back are termed as the reflected waves.
22. Which among the following is the incorrect law of reflection
- The incident wave, the normal to the reflecting surface and the reflected wave at the point of incidence lie in different plane
- The angle of incidence ∠i is greater than the angle of reflection ∠r.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Like light waves, sound waves also obey some fundamental laws of reflection. The following two laws of reflection are applicable to sound waves as well.
1. The incident wave, the normal to the reflecting surface and the reflected wave at the point of incidence lie in the same plane.
2. The angle of incidence ∠i is equal to the angle of reflection ∠r.
23. A perpendicular line drawn at the point of incidence is called the ____
- Medium
- Normal
- Conjunction
- Tune
Explanation
A perpendicular line drawn at the point of incidence is called the normal. The angle which the incident sound wave makes with the normal is called the angle of incidence, ‘i’. The angle which the reflected wave makes with the normal is called the angle of reflection, ‘r’.
24. Which among the following fort there is Clapping portico which is a series of arches on one side, each smaller than the preceding one?
- Gwalior Fort
- Golconda Fort
- Amber Fort
- Bidar Fort
Explanation
The Clapping portico in Golconda Fort is a series of arches on one side, each smaller than the preceding one. So, a sound wave generated under the dome would get compressed and then bounce back amplified sufficiently to reach a considerable distance.
25. Which among the following statement is incorrect
- A longitudinal wave travels in a medium in the form of compressions and rarefactions. Suppose a compression travelling in air from left to right reaches a rigid wall. The compression exerts a force F on the rigid wall.
- In turn, the wall exerts an equal and opposite reaction R = – F on the air molecules. This results in a compression near the rigid wall. Thus, a compression travelling towards the rigid wall is reflected back as a compression. That is the direction of compression is reversed.
- Only 1
- Only 2
- Both 1 and 2
- None
26. Who first observed that the frequency of the sound as received by a listener is different from the original frequency produced by the source whenever there is a relative motion between the source and the listener?
- James Maxwell
- Edwin Hubble
- Christian Doppler
- Ezra Pound
Explanation
Christian Doppler (1803-1853), an Austrian Mathematician and Physicist first observed that the frequency of the sound as received by a listener is different from the original frequency produced by the source whenever there is a relative motion between the source and the listener. This is known as Doppler effect.
27. Which among the following is the possibility of doppler effect relative motion?
- The listener moves towards or away from a stationary source. The source moves towards or away from a stationary listener.
- Both source and listener move towards or away from one other. The medium moves when both source and listener are at rest.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Doppler effect relative motion could be due to various possibilities as follows: (i) The listener moves towards or away from a stationary source (ii) The source moves towards or away from a stationary listener (iii) Both source and listener move towards or away from one other (iv) The medium moves when both source and listener are at rest.
28. Which among the following statement is correct
- Let S and L be the source and the listener moving with velocities vS and vL respectively. Consider the case of source and listener moving towards each other. As the distance between them decreases, the apparent frequency will be more than the actual source frequency.
- Let n and n’ be the frequency of the sound produced by the source and the sound observed by the listener respectively. Then, the expression for the apparent frequency n’ is
Here, v is the velocity of sound waves in the given medium. Let us consider different possibilities of motions of the source and the listener.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Let n and n’ be the frequency of the sound produced by the source and the sound observed by the listener respectively. Then, the expression for the apparent frequency n’ is
Here, v is the velocity of sound waves in the given medium. Let us consider different possibilities of motions of the source and the listener.
29. Which among the following Expression for apparent frequency is wrong based Position of source and listener
- Both source and listener move They move towards each other
- Both source and listener move They move away from each other n’ =
- Both source and listener move They move one behind the other. Source follows the listener
n’ = (
- Both source and listener move They move one behind the other Listener follows the source
n’ = n
- Both 1 and 2
- Both 1 and 3
- Both 3 and 4
- Both 2 and 4
Explanation


30. A source and listener are both moving towards each other with a speed v/10 where v is the speed of sound. If the frequency of the note emitted by the source is f, what will be the frequency heard by the listener?
- n’ = 1.22 f
- n’ = 2.44 f
- n’ = 3.66 f
- n’ =4.88 f
Explanation
When source and listener are both moving towards each other, the apparent frequency
is n
n’= (11/9) f
n’= 1.22 f
31. Which among the following Expression for apparent frequency is correct based Position of source and listener?
- Source at rest. Listener moves towards the source n’ =
- Source at rest. Listener moves away from the source n’ =
- Listener at rest. Source moves towards the listener n’ =
- Listener at rest Source moves away from the listener n’ =
- Both 1 and 2
- Both 1 and 3
- Both 3 and 4
- Both 2 and 4
Explanation


32. At what speed should a source of sound move away from a stationary observer so that observer finds the apparent frequency equal to half of the original frequency?
- Vs =2 V
- Vs = 1.5V
- Vs = V
- Vs = 0.5V
Explanation
n’ =
=
Vs = V.
33. Which among the following circumstance there is a doppler effect occurs
- When source (S) and listener (L) both are at rest
- When S and L move in a opposite way that distance between them remains change continuously.
- When source S and L are moving in mutually perpendicular directions.
- If the source is situated at the centre of the circle along which the listener is moving.
Explanation
When S and L move in such a way that distance between them remains constant.
34. Which among the following statement is correct
- An electromagnetic wave is emitted by a source attached to a police car. The wave is reflected by a moving vehicle, which acts as a moving source. There is a shift in the frequency of the reflected wave. From the frequency shift, the speed of the car can be determined. This helps to track the over speeding vehicles.
- The frequency of radio waves emitted by a satellite increase as the satellite passes away from the Earth. By measuring the change in the frequency of the radio waves, the location of the satellites is studied.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
The frequency of radio waves emitted by a satellite decreases as the satellite passes away from the Earth. By measuring the change in the frequency of the radio waves, the location of the satellites is studied.
35. In what by measuring the change in the frequency between the sent signal and received signal, the speed of marine animals and submarines can be determined?
- SONAR
- RADAR
- LIDAR
- None of the above
Explanation
In SONAR, by measuring the change in the frequency between the sent signal and received signal, the speed of marine animals and submarines can be determined.
36. In what radio waves are sent, and the reflected waves are detected by the receiver of the station?
- SONAR
- RADAR
- LIDAR
- AIDAR
Explanation
In RADAR, radio waves are sent, and the reflected waves are detected by the receiver of the RADAR station. From the frequency change, the speed and location of the aeroplanes and aircrafts are tracked.
37. The medium in which the velocity of sound increases compared to another medium is called ____________
- Denser medium
- Closer medium
- Rarer medium
- Pointer medium
Explanation
The medium in which the velocity of sound increases compared to another medium is called rarer medium. (Water is rarer compared to air for sound).
38. The medium in which the velocity of sound decreases compared to another medium is called ____
- Denser medium
- Closer medium
- Rarer medium
- Pointer medium
Explanation
The medium in which the velocity of sound decreases compared to another medium is called denser medium. (Air is denser compared to water for sound).
39. Which among the following statement is correct
- Consider a wave travelling in a solid medium striking on the interface between the solid and the air. The compression exerts a force F on the surface of the rarer medium. As, a rarer medium has larger resistance for deformation, the surface of separation is pushed forward.
- As the particles of the rarer medium are free to move, a rarefaction is produced at the interface. Thus, a compression is reflected as a rarefaction and a rarefaction travels from right to left.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Consider a wave travelling in a solid medium striking on the interface between the solid and the air. The compression exerts a force F on the surface of the rarer medium. As a rarer medium has smaller resistance for any deformation, the surface of separation is pushed backwards.
40. In which surface sound from one focus will always be reflected to the other focus, no matter where it strikes the wall?
- Convex surface
- Concave surface
- Parabolic surface
- Elliptical surface
Explanation
In elliptical surfaces, sound from one focus will always be reflected to the other focus, no matter where it strikes the wall. This principle is used in designing whispering halls. In a whispering hall, the speech of a person standing in one focus can be heard clearly by a listener standing at the other focus.
41. One of the famous whispering galleries is in St. Paul’s cathedral church is located in which among the following city?
- Paris
- Berlin
- London
- Cape town
Explanation
One of the famous whispering galleries is in St. Paul’s cathedral church in London. It is built with elliptically shaped walls. When a person is talking at one focus, his voice can be heard distinctly at the other focus. It is due to the multiple reflections of sound waves from the curved walls.
42. A source producing a sound of frequency 90 Hz is approaching a stationary listener with a speed equal to (1/10) of the speed of sound. What will be the frequency heard by the listener?
- n’ = 9 Hz
- n’ = 90 Hz
- n’ = 100 Hz
- n’ = 110 Hz
Explanation
When the source is moving towards the stationary listener, the expression for apparent frequency is
n’ = = = ()n
= × 90 = 100 Hz.
43. From which surface, the reflected waves travel in a direction, according to the law of reflection thus intensity of the reflected wave is neither decreased nor increased?
- Plane surface
- Curved surface
- Convex surface
- Concave surface
Explanation
When sound waves are reflected from a plane surface, the reflected waves travel in a direction, according to the law of reflection. The intensity of the reflected wave is neither decreased nor increased.
44. Which surface are used when it is required to focus the sound at a particular point?
- Plane surface
- Parabolic surface
- Convex surface
- None of the above
Explanation
Parabolic surfaces are used when it is required to focus the sound at a particular point. Hence, many halls are designed with parabolic reflecting surfaces.
45. Which among the following statement is incorrect
- When the sound waves are reflected from the curved surfaces, the intensity of the reflected waves is changed. When reflected from a convex surface, the reflected waves are diverged out and the intensity is increased.
- When sound is reflected from a concave surface, the reflected waves are increased and dispersed at various point. So, the intensity of reflected waves is concentrated at various point.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
When the sound waves are reflected from the curved surfaces, the intensity of the reflected waves is changed. When reflected from a convex surface, the reflected waves are diverged out and the intensity is decreased. When sound is reflected from a concave surface, the reflected waves are converged and focused at a point. So, the intensity of reflected waves is concentrated at a point.
46. A source producing a sound of frequency 500 Hz is moving towards a listener with a velocity of 30 m s–1. The speed of the sound is 330 m s–1. What will be the frequency heard by listener?
- n’ = 450 Hz
- n’ = 550 Hz
- n’ = 650 Hz
- n’ = 500 Hz
Explanation
When the source is moving towards the stationary listener, the expression for apparent frequency is
n’ =
n’ =
= 550 Hz.
47. Which is the sound reproduced due to the reflection of the original sound from various rigid surfaces such as walls, ceilings, surfaces of mountains, etc.
- LIDAR
- Echo
- All the above
Explanation
An echo is the sound reproduced due to the reflection of the original sound from various rigid surfaces such as walls, ceilings, surfaces of mountains, etc. If you shout or clap near a mountain or near a reflecting surface, like a building you can hear the same sound again. The sound, which you hear is called an echo. It is due to the reflection of sound.
48. Which among the following Conditions necessary for hearing echo is incorrect
- The persistence of hearing for human ears is 0.5 second. This means that you can hear two sound waves clearly, if the time interval between the two sounds is at least 0.5 s. Thus, the minimum time gap between the original sound and an echo must be 0.5 s.
- The above criterion can be satisfied only when the distance between the source of sound and the reflecting surface would satisfy the following equation:
Velocity (v) = =
d = , since, t = 0.1 second, then d = =
Thus, the minimum distance required to hear an echo is 1/20th part of the magnitude of the velocity of sound in air. If you consider the velocity of sound as 344 m s–1, the minimum distance required to hear an echo is 17.2 m.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
The persistence of hearing for human ears is 0.1 second. This means that you can hear two sound waves clearly, if the time interval between the two sounds is a least 0.1 s. Thus, the minimum time gap between the original sound and an echo must be 0.1 s.
49. Which among the following statement is correct
- Some animals communicate with each other over long distances and also locate objects by sending the sound signals and receiving the echo as reflected from the targets. Echo is used to determine the velocity of sound waves in any medium.
- The principle of echo is used in obstetric ultrasonography, which is used to create real-time visual images of the developing embryo or fetus in the mother’s uterus. This is a safe testing tool, as it does not use any harmful radiations.
- Only 1
- Only 2
- Both 1 and 2
- None
50. A source of sound is moving with a velocity of 50 m s–1 towards a stationary listener. The listener measures the frequency of the source as 1000 Hz. what will be the apparent frequency of the source when it is moving away from the listener after crossing him? (velocity of sound in the medium is 330 m s–1)
- n’ = 848.48 Hz.
- n’ = 736.84 Hz
- n’ = 682.15 Hz
- n’ = 562.52 Hz
Explanation
When the source is moving towards the stationary listener, the expression for apparent frequency is
n’ =
1000 =
n =
The actual frequency of the sound is 848.48 Hz. When the source is moving away from the stationary listener, the expression for apparent frequency is
n’ =
=
= 736.84 Hz.
51. Which among he following statement is incorrect regarding Measuring velocity of sound by echo method
- Measure the distance ‘d’ between the source of sound pulse and the reflecting surface using the measuring tape. The receiver is also placed adjacent to the source. A sound pulse is emitted by the source.
- The stopwatch is used to note the time interval between the instant at which the sound pulse is sent and the instant at which the echo is received by the receiver. Note the time interval as ‘t’. Repeat the experiment for three or four times. The average time taken for the given number of pulses is calculated.
- The sound pulse emitted by the source travels a total distance of d while travelling from the source to the wall and then back to the receiver. The time taken for this has been observed to be ‘t’. Hence,
Speed of sound =
- Both 1 and 2
- Both 2 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
The sound pulse emitted by the source travels a total distance of 2d while travelling from the source to the wall and then back to the receiver. The time taken for this has been observed to be ‘t’. Hence,
Speed of sound =
52. Which among the following statement is correct
- Sound board are basically square surfaces (plain), which are used in auditoria and halls to improve the quality of sound. This board is placed such that the speaker is at the focus of the plane surface. The sound of the speaker is reflected towards the audience thus improving the quality of sound heard by the audience.
- A megaphone is a horn-shaped device used to address a small gathering of people. Its one end is wide and the other end is narrow. When a person speaks at the narrow end, the sound of his speech is concentrated by the multiple reflections from the walls of the tube. Thus, his voice can be heard loudly over a long distance.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Sound board are basically curved surfaces (concave), which are used in auditoria and halls to improve the quality of sound. This board is placed such that the speaker is at the focus of the concave surface. The sound of the speaker is reflected towards the audience thus improving the quality of sound heard by the audience.
53. Which among the following is a hearing aid, which is useful by people who have difficulty in hearing?
- Ear spinner
- Ear copper
- Ear pole
- Ear trumpet
Explanation
Ear trumpet is a hearing aid, which is useful by people who have difficulty in hearing. In this device, one end is wide and the other end is narrow. The sound from the sources fall into the wide end and are reflected by its walls into the narrow part of the device. This helps in concentrating the sound and the sound enters the ear drum with more intensity. This enables a person to hear the sound better.
10th Science Lesson 6 Questions in English
6] Nuclear Physics
1. Which Greek philosopher in 400 BC(BCE) believed that matter is made up of tiny indestructible units called atoms?
- Parmenides
- Pythagoras
- Democritus
- Plato
Explanation
Humans are very much interested in knowing about atoms. Things around us are made up of atoms. A Greek Philosopher ‘Democritus’ in 400 BC(BCE) believed that matter is made up of tiny indestructible units called atoms.
2. In 1803, who considered that elements consist of atoms, which are identical in nature?
- John Dalton
- J J Thomson
- James Chadwick
- Henri Becquerel
Explanation
In 1803, John Dalton considered that elements consist of atoms, which are identical in nature.
3. In 1896, which French physicist finished his research for the week and stored a certain amount of uranium compound away in a drawer for the week end?
- Rutherford
- Henri Becquerel
- J J Thomson
- James Chadwick
Explanation
In 1896, French physicist Henri Becquerel finished his research for the week and stored a certain amount of uranium compound away in a drawer for the week end. By chance, an unexposed photographic plate was also stored in the same drawer. After a week he returned and noticed that the film had been exposed to some radiation. He discovered that he could reproduce the effect whenever he placed uranium near a photographic film.
4. The uranium radiated something that could affect a photographic plate. This phenomenon was called as ______
- Optimist
- Radioactivity
- Conductivity
- Relativity
Explanation
The uranium radiated something that could affect a photographic plate. This phenomenon was called as Radioactivity. Uranium was identified to be a radioactive element.
5. Which polish physicist detected radioactivity in ‘Pitchblende’, a tiny black substance?
- Gerty Theresa and Carl Ferdinand Cori
- Antoine and Marie-Anne Lavoisier
- Fredric Joliot and Irene Joliot Curie
- Marie Curie and Pierre Curie
Explanation
The Polish physicist Marie Curie and her husband Pierre Curie detected radioactivity in ‘Pitchblende’, a tiny black substance. They were not surprised at the radioactivity of pitchblende, which is known as an ore of uranium. Later, they discovered that the radiation was more intense from pure uranium. Also, it was found that the pitchblende had less concentration of uranium.
6. Marie Curie and her husband Pierre Curie concluded that some other substance was present in pitchblende. After separating this new substance, they discovered that it emitted radiations spontaneously like uranium. They named this new substance as ______
- Thorium
- Plutonium
- Radium
- Francium
Explanation
Marie Curie and her husband Pierre Curie concluded that some other substance was present in pitchblende. After separating this new substance, they discovered that it had unknown chemical properties and it also emitted radiations spontaneously like uranium. They named this new substance as ‘Radium’. The radioactive elements emit harmful radioactive radiations like alpha rays or beta rays or gamma rays.
7. Which among the following statement is correct
- The nucleus of some elements is unstable. Such nuclei undergo nuclear decay and get converted into more stable nuclei. During this nuclear reaction, these nuclei emit certain harmful radiations and elementary particles.
- The phenomenon of nuclear decay of certain elements with the emission of radiations like alpha, beta, and gamma rays is called ‘radioactivity’ and the elements, which undergo this phenomenon are called ‘radioactive elements’.
- Only 1
- Only 2
- Both 1 and 2
- None
8. The elements whose atomic number is more than what undergo spontaneous radioactivity?
- 70
- 74
- 78
- 82
Explanation
The elements such as uranium and radium undergo radioactivity and emit the radiations on their own without any human intervention. This phenomenon of spontaneous emission of radiation from certain elements on their own is called ‘natural radioactivity’. The elements whose atomic number is more than 82 undergo spontaneous radioactivity.
9. Which among the following is not the radioactive element?
- Gallium
- Technetium
- Nobelium
- Astatine
Explanation
Gallium is not the radioactive element.
10. Which among the following radioactive element have been identified as radioactive substances with atomic number less than 82?
- Curium
- Thorium
- Technetium
- Astatine
Explanation
There are only two elements, which have been identified as radioactive substances with atomic number less than 82. They are technetium (Tc) with atomic number 43 and promethium (Pm) with atomic number 61.
11. Who among the following first discovered ‘artificial radioactivity’ or ‘man-made radioactivity?
- Gerty Theresa and Carl Ferdinand Cori
- Antoine and Marie-Anne Lavoisier
- Fredric Joliot and Irene Curie
- Marie Curie and Pierre Curie
Explanation
The phenomenon by which even light elements are made radioactive, by artificial or induced methods, is called ‘artificial radioactivity’ or ‘man-made radioactivity’. This kind of radioactivity was discovered by Irene Curie and Fredric Joliot in 1934.
12. Which among the following statement is correct
- Artificial radioactivity is induced in certain lighter elements like boron, aluminium etc., by bombarding them with radiations such as ‘alpha particles’ emitted during the natural radioactivity of uranium. This also results in the emission of invisible radiations and elementary particles.
- During such a disintegration, the nucleus which undergoes disintegration is called ‘daughter nucleus’ and that which is produced after the disintegration is called a ‘parent nucleus’. When the projectile hits the parent nucleus, it is converted into an unstable nucleus, which in turn decays spontaneously emitting the daughter nucleus along with an ejected particle.
- If you denote the parent and daughter nuclei as X and Y respectively, then the nuclear disintegration is represented as follows: X (P, E) Y. Here, P and E represent the projectile particle and ejected particle respectively.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
During such a disintegration, the nucleus which undergoes disintegration is called ‘parent nucleus’ and that which is produced after the disintegration is called a ‘daughter nucleus’. When the projectile hits the parent nucleus, it is converted into an unstable nucleus, which in turn decays spontaneously emitting the daughter nucleus along with an ejected particle.
13. How many radioactive substances discovered so far?
- 24
- 27
- 29
- 32
Explanation
There have been 29 radioactive substances discovered so far. Most of them are rare earth metals and transition metals.
14. The particle, which is used to induce the artificial disintegration is termed as ____
- Projectile
- Ejectile
- Conserve
- Cone
Explanation
The particle, which is used to induce the artificial disintegration is termed as projectile and the particle which is produced after the disintegration is termed as ejected particle.
15. Complete the following equation: 4Be9 + 2He 4 ???? ____
- 6C11 + 0 n2
- 6C12 + 0 n1
- 6C9 + 0 n4
- 6C10 + 0 n3
Explanation
4Be9 + 2He 4 ???? 6C13*
6C13* ???? 6C12 + 0n1
In the above nuclear reaction, 6 C13* is unstable and is radioactive. This reaction can be represented as 4Be9 (α, n) 6C12.
4Be9 + 2He4 ????6C12 + 0n1
16. Which among the following is not the property of Natural radioactivity?
- Emission of radiation due to self-disintegration of a nucleus.
- Alpha, beta and gamma radiations are emitted
- It is a spontaneous process.
- This can be controlled.
Explanation
Natural radioactivity properties are 1. Emission of radiation due to self-disintegration of a nucleus, 2. Alpha, beta and gamma radiations are emitted, 3. It is a spontaneous process, 4. Exhibited by elements with atomic number more than 83 and 5. This cannot be controlled.
17. Which among the following is not the units of Radioactivity?
- Lavoisier
- Curie
- Rutherford
- Becquerel
Explanation
The units of Radioactivity are 1. Curie, 2. Rutherford, 3. Becquerel and 4. Roentgen.
18. Which among the following is the SI unit of Radioactivity?
- Curie
- Rutherford
- Becquerel
- Roentgen
Explanation
The SI unit of radioactivity is becquerel. It is defined as the quantity of one disintegration per second.
19. Which is defined as the quantity of radioactive substance which produces a charge of 2.58 × 10-4 coulomb in 1 kg of air under standard conditions of pressure, temperature and humidity?
- One curie
- One Rutherford
- One Becquerel
- One Roentgen
Explanation
The radiation exposure of γ and x-rays is measured by another unit called roentgen. One roentgen is defined as the quantity of radioactive substance which produces a charge of 2.58 × 10-4 coulomb in 1 kg of air under standard conditions of pressure, temperature and humidity.
20. Which is defined as the quantity of a radioactive substance, which produces 106 disintegrations in one second?
- Curie
- Rutherford
- Becquerel
- Roentgen
Explanation
Rutherford (Rd) is another unit of radioactivity. It is defined as the quantity of a radioactive substance, which produces 106 disintegrations in one second.
21. Which among the following is not the property of Artificial radioactivity?
- Emission of radiation due to disintegration of a nucleus through induced process.
- Mostly elementary particles such as neutron, positron, etc. are emitted.
- It is a spontaneous process.
- This can be controlled.
Explanation
The property of Artificial radioactivity is 1. Emission of radiation due to disintegration of a nucleus through induced process, 2. Mostly elementary particles such as neutron, positron, etc. are emitted, 3. It is an induced process, 4. Exhibited by elements with atomic number less than 83 and 5. This can be controlled.
22. Which is the traditional unit of radioactivity?
- Curie
- Rutherford
- Becquerel
- Roentgen
Explanation
Curie is the traditional unit of radioactivity. It is defined as the quantity of a radioactive substance which undergoes 3.7 × 1010 disintegrations in one second. This is actually close to the activity of 1 g of radium 226. 1 curie = 3.7 × 1010 disintegrations per second.
23. Which among the following particles is not comprised in radiations emitted by radioactive nucleus?
- Alpha
- Beta
- Gamma
- Delta
Explanation
When a radioactive nucleus undergoes radioactivity, it emits harmful radiations. These radiations are usually comprised of any of the three types of particles. They are alpha(α), beta (β) and gamma(γ) rays.
24. Who among the following German chemist discovered Uranium in a mineral called pitchblende?
- William Gregor
- Carl Mosander
- Martin Klaproth
- Hamphry Davy
Explanation
Uranium, named after the planet Uranus, was discovered by Martin Klaproth, a German chemist in a mineral called pitchblende.
25. Which among the following is not the property of α rays?
- Helium nucleus (2He4) consisting of two protons and two neutrons.
- Positively charged particles. Charge of each alpha particle = +2e
- Penetrating power is greater than that of β rays. They can penetrate through a thin metal foil.
- Deflected by both the fields. (in accordance with Fleming’s left hand rule)
Explanation
Low penetrating power (even stopped by a thick paper). Ionising power is 100 time greater than β rays and 10,000 times greater than γ rays.
26. Which among the following is not the property of γ rays?
- They are electromagnetic waves consisting of photons.
- Negatively charged particles. Charge of each beta particle = –e
- They have a very high penetrating power greater than that of β rays. They can penetrate through thick metal blocks.
- They are not deflected by both the fields
Explanation
Neutral particles. Charge of each gamma particle = zero. Very less ionization power
27. Which among the following is not the property of β rays?
- They are electrons (-1e0), basic elementary particle in all atoms.
- Negatively charged particles. Charge of each beta particle = –e
- Deflected by only one field; but the direction of deflection is same as that of alpha rays. (in accordance with Fleming’s left hand rule)
- They travel with the speed of light.
Explanation
Deflected by both the fields; but the direction of deflection is opposite to that for alpha rays. (in accordance with Fleming’s left hand rule). Penetrating power is greater than that of α rays. They can penetrate through a thin metal foil.
28. Which among the following statement is incorrect
- α rays speed ranges from 1/10 to 1/20 times the speed of light
- γ rays speed can go up to 9/10 times the speed of light.
- β rays travel with the speed of light.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
β rays speed can go up to 9/10 times the speed of light. γ rays travel with the speed of light.
29 Complete the following equation: 92U238 ???? _____
- 90Th234 +2He 4
- 90Pm234 +2He 4
- 90Np234 +2He 4
- 90Rf234 +2He 4
Explanation
92U238 ???? 90Th234 +2He4
30. Which among the following statement is correct regarding Gamma decay
- In the radioactive nucleus the atomic number is greater than the mass number
- In the radioactive nucleus the mass number is greater than the atomic number
- The atomic number and mass number of the radioactive nucleus remain the same
- None of the above
Explanation
In a γ – decay, only the energy level of the nucleus changes. The atomic number and mass number of the radioactive nucleus remain the same.
31. When Uranium (U238) decays to thorium (Th234) it emits which particle?
- Gamma particle
- Beta particle
- Alpha particle
- All the above
Explanation
A nuclear reaction in which an unstable parent nucleus emits an alpha particle and forms a stable daughter nucleus, is called ‘alpha decay’. E.g.: Decay of uranium (U238) to thorium (Th234) with the emission of an alpha particle.
92U238 ???? 90Th234 +2He4 (α – decay)
In α – decay, the parent nucleus emits an α particle and so it is clear that for the daughter nucleus, the mass number decreases by four and the atomic number decreases by two.
32. Complete the following equation: 15P32 ???? _____
- 16Cf32 + -1e0
- 16S32 + -1e0
- 16F32 + -1e0
- 16B32 + -1e0
Explanation
A nuclear reaction, in which an unstable parent nucleus emits a beta particle and forms a stable daughter nucleus, is called ‘beta decay’. E.g.: Beta decay of phosphorous.
15P32 ???? 16S32 + -1e0
In β – decay there is no change in the mass number of the daughter nucleus but the atomic number increases by one.
33. Which German scientists discovered that when a uranium nucleus is bombarded with a neutron, it breaks up into two smaller nuclei of comparable mass along with the emission of a few neutrons and energy?
- Otto Hahn and F. Strassman
- Irene Curie and F. Joliot
- Antoine and Marie-Anne Lavoisier
- Adair Crawford and Humphry Davy
Explanation
In 1939, German Scientist Otto Hahn and F. Strassman discovered that when a uranium nucleus is bombarded with a neutron, it breaks up into two smaller nuclei of comparable mass along with the emission of a few neutrons and energy.
34. The process of breaking (splitting) up of a heavier nucleus into two smaller nuclei with the release of a large amount of energy and a few neutrons is called ____
- Nuclear fission
- Nuclear fusion
- Nuclear Spike
- All the above
Explanation
The process of breaking (splitting) up of a heavier nucleus into two smaller nuclei with the release of a large amount of energy and a few neutrons is called ‘nuclear fission’.
35. Complete the following Equation: 92U235 + 0n1 ???? _____
- 92U235 + 0n1 ???? 56Cf141 + 36Rn92 + 30n1 + Q
- 92U235 + 0n1 ???? 56Pm141 + 36Np92 + 30n1 + Q
- 92U235 + 0n1 ???? 56Ba141 + 36Kr92 + 30n1 + Q
- 92U235 + 0n1 ???? 56Po141 + 36Tc92 + 30n1 + Q
Explanation
Nuclear fission of a uranium nucleus (U235): 92U235 + 0n1 ???? 56Ba141 + 36Kr92 + 30n1 + Q.
The average energy released in each fission process is about 3.2 × 10-11 J. Nuclear fission is pictorially represented.
36. Which among the following statement is correct
- A fissionable material is a radioactive element, which undergoes fission in a sustained manner when it absorbs a neutron. It is also termed as ‘fissile material’. E.g.: U235, plutonium (Pu239 and Pu241)
- All isotopes of uranium undergo nuclear fission when they absorb a neutron. For example, natural uranium consists of 99.28 % of 92U238 and 0.72 % of 92U235. Of these two, U238 and U235 undergoes fission at different rate.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
All isotopes of uranium do not undergo nuclear fission when they absorb a neutron. For example, natural uranium consists of 99.28 % of 92U238 and 0.72 % of 92U235. Of these two, U238 does not undergo fission whereas U235 undergoes fission. Hence, U235 is a fissionable material and U238 is non fissionable.
37. There are some radioactive elements, which can be converted into fissionable material. They are called as _____
- Coil material
- Morse material
- Reserve material
- Fertile material
Explanation
There are some radioactive elements, which can be converted into fissionable material. They are called as fertile materials. E.g.: Uranium-238, Thorium-232, Plutonium-240.
38. A uranium nucleus (U-235) when bombarded with a neutron undergoes fission producing three neutrons. These three neutrons in turn can cause fission in three other uranium nuclei present in the sample, thus producing nine neutrons. This is known as _____
- Critical reaction
- Mass reaction
- Liquid reaction
- Chain reaction
Explanation
A uranium nucleus (U-235) when bombarded with a neutron undergoes fission producing three neutrons. These three neutrons in turn can cause fission in three other uranium nuclei present in the sample, thus producing nine neutrons. These nine neutrons in turn may produce twenty-seven neutrons and so on. This is known as ‘chain reaction’. A chain reaction is a self-propagating process in which the number of neutrons goes on multiplying rapidly almost in a geometrical progression.
39. How many kinds of chain reactions are possible in Nuclear fission?
- One
- Two
- Three
- Six
Explanation
Two kinds of chain reactions are possible. They are: (i) controlled chain reaction and (ii) uncontrolled chain reaction.
40. Which among the following statement is incorrect
- In the controlled chain reaction, the number of neutrons released is maintained to be one. This is achieved by absorbing the extra neutrons with a neutron absorber leaving only one neutron to produce further fission. Thus, the reaction is sustained in a controlled manner.
- The energy released due to a controlled chain reaction can be utilized for constructive purposes. Controlled chain reaction is used in the atom bomb to produce an explosion energy in a sustained manner.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
The energy released due to a controlled chain reaction can be utilized for constructive purposes. Controlled chain reaction is used in a nuclear reactor to produce energy in a sustained and controlled manner.
41. Which kind of chain reaction is used in Atom bomb?
- Controlled chain reaction
- Uncontrolled chain reaction
- Both Controlled and Uncontrolled
- Semi Controlled chain reaction
Explanation
In the uncontrolled chain reaction, the number of neutrons multiplies indefinitely and causes fission in a large amount of the fissile material. This results in the release of a huge amount of energy within a fraction of a second. This kind of chain reaction is used in the atom bomb to produce an explosion.
42. Which among the following statement is correct?
- During a nuclear fission process, about 2 to 3 neutrons are released. But all these neutrons may not be available to produce further fission. Some of them may escape from the system, which is termed as ‘leakage of neutrons’ and some may be absorbed by the non-fissionable materials present in the system.
- These two factors lead to the loss of neutrons. To sustain the chain reaction, the rate of production of neutrons due to nuclear fission must be more than the rate of its loss. This can be achieved only when the size (i.e., mass) of the fissionable material is equal to a certain optimum value. This is known as ‘critical mass’.
- The minimum mass of a fissile material necessary to sustain the chain reaction is called ‘critical mass (m c)’. It depends on the nature, density and the size of the fissile material.
- Both 1 and 2
- Both 1 and 3
- Both 3 and 3
- All 1, 2 and 3
43. If the mass of the fissile material is less than the critical mass, it is termed as _____
- Micro critical
- Subcritical
- Super critical
- Sonic critical
Explanation
If the mass of the fissile material is less than the critical mass, it is termed as ‘subcritical’.
44. If the mass of the fissile material is more than the critical mass, it is termed as ____
- Sonic critical
- Scope critical
- Super critical
- All the above
Explanation
If the mass of the fissile material is more than the critical mass, it is termed as ‘supercritical’.
45. Which among the following statement is correct
- The atom bomb is based on the principle of uncontrolled chain reaction. In an uncontrolled chain reaction, the number of neutrons and the number of fission reactions multiply almost in a geometrical progression. This releases a huge amount of energy in a very small time interval and leads to an explosion.
- An atom bomb consists of a piece of fissile material whose mass is subcritical. This piece has a cylindrical void. It has a cylindrical fissile material which can fit into this void and its mass is also subcritical. When the bomb has to be exploded, this cylinder is injected into the void using a conventional explosive. Now, the two pieces of fissile material join to form the supercritical mass, which leads to an explosion.
- During this explosion tremendous amount of energy in the form of heat, light and radiation is released. A region of very high temperature and pressure is formed in a fraction of a second along with the emission of hazardous radiation like γ rays, which adversely affect the living creatures. This type of atom bombs was exploded in 1947 at Hiroshima and Nagasaki in Japan during the World War II.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
During this explosion tremendous amount of energy in the form of heat, light and radiation is released. A region of very high temperature and pressure is formed in a fraction of a second along with the emission of hazardous radiation like γ rays, which adversely affect the living creatures. This type of atom bombs was exploded in 1945 at Hiroshima and Nagasaki in Japan during the World War II.
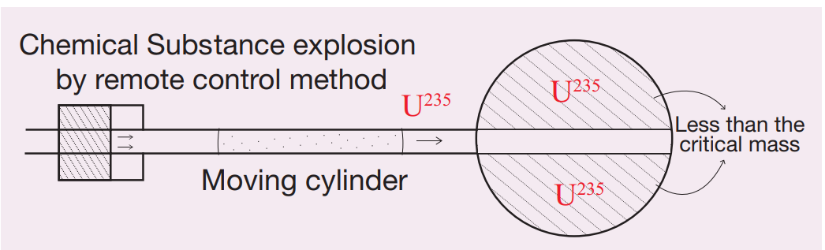
46. Which is the unit used in nuclear physics to measure the energy of small particles?
- Mass Valent
- Critical Joule
- Electron Volte
- Ohm Electron
Explanation
Electron Volt (eV) is the unit used in nuclear physics to measure the energy of small particles. It is nothing but the energy of one electron when it is accelerated using an electric potential of one volt. 1eV = 1.602 × 10-19 joule. 1 million electron volt = 1 MeV = 106 eV (mega electron volt). The energy released in a nuclear fission process is about 200 MeV.
47. The energy can be produced when two lighter nuclei combine to form a heavier nucleus. This phenomenon is known as _____
- Nuclear fission
- Nuclear fusion
- Nuclear Spike
- All the above
Explanation
The energy can be produced when two lighter nuclei combine to form a heavier nucleus. This phenomenon is known as nuclear fusion.
48. Nuclear fusion reaction. 1 H2 + 1 H2 ????2 He4 + Q (Energy). Here, 1H2 represents an isotope of hydrogen known as _____
- Neuterium
- Deuterium
- Poterium
- Santerium
Explanation
Here, 1 H2 represents an isotope of hydrogen known as ‘deuterium’. The average energy released in each fusion reaction is about 3.84 × 10-12 J.
49. The mass of the daughter nucleus formed during a nuclear reaction (fission and fusion) is lesser than the sum of the masses of the two parent nuclei. This difference in mass is called ____
- Mass effect
- Mass corrupt
- Mass retreat
- Mass defect
Explanation
The mass of the daughter nucleus formed during a nuclear reaction (fission and fusion) is lesser than the sum of the masses of the two parent nuclei. This difference in mass is called mass defect. This mass is converted into energy, according to the mass-energy equivalence.
50. Who discovered cathode rays, known as electrons, experimentally?
- John Dalton
- J J Thomson
- Goldstein
- Rutherford
Explanation
J J Thomson discovered cathode rays, known as electrons, experimentally.
51. Who discovered positive rays, which were named as protons by Rutherford?
- John Dalton
- James Chadwick
- Goldstein
- Rutherford
Explanation
Goldstein discovered positive rays, which were named as protons by Rutherford.
52. Who proposed the relation between mass and energy i.e., E = mc2?
- Newton
- Einstein
- Pascal
- Marie Curie
Explanation
The concept of mass-energy equivalence was proposed by Einstein in 1905. It stated that mass can be converted into energy and vice versa. The relation between mass and energy proposed by Einstein is E = mc2 where c is the velocity of light in vacuum and is equal to 3 × 108 m s–1.
53. The nuclear bomb that was dropped in Hiroshima during World War II was called as ____
- Fat man
- Small man
- Little boy
- Old boy
Explanation
The nuclear bomb that was dropped in Hiroshima during World War II was called as ‘Little boy’. It was a gun-type bomb which used a uranium core.
54. Nuclear fusion is possible only at an extremely high temperature of the order of 107 to 109 K and a high pressure to push the hydrogen nuclei closer to fuse with each other. Hence, it is named as ___________
- Thermonuclear reaction
- Subatomic reaction
- Nerve reaction
- Nano nuclear reaction
Explanation
Nuclear fusion is possible only at an extremely high temperature of the order of 107 to 109 K and a high pressure to push the hydrogen nuclei closer to fuse with each other. Hence, it is named as ‘Thermonuclear reaction’.
55. Which among the following statement is correct
- Nuclear fusion is the combination of two lighter nuclei. The charge of both nuclei is negative. According to electrostatic theory, when they come closer, they tend to repel each other.
- This repulsive force will be overcome by the kinetic energy of the nuclei at higher temperature of the order of 107 to 109 K.
- Only 1
- Only 2
- Both 1 and 2
- None
Explanation
Nuclear fusion is the combination of two lighter nuclei. The charge of both nuclei is positive. According to electrostatic theory, when they come closer, they tend to repel each other.
56. The stars like our Sun emit a large amount of energy in the form of light and heat. This energy is termed as ____
- Constellation energy
- Stellar energy
- Basal energy
- All the above
Explanation
The stars like our Sun emit a large amount of energy in the form of light and heat. This energy is termed as the stellar energy. All stars contain a large amount of hydrogen. The surface temperature of the stars is very high which is sufficient to induce fusion of the hydrogen nuclei. Fusion reaction that takes place in the cores of the Sun and other stars results in an enormous amount of energy, which is called as ‘stellar energy. Thus, nuclear fusion or thermonuclear reaction is the source of light and heat energy in the Sun and other stars.
57. The bomb, which was subsequently dropped over Nagasaki was called as ___
- Fat man
- Small man
- Little boy
- Old boy
Explanation
The bomb, which was subsequently dropped over Nagasaki was called as ‘Fat man’. It was an explosion type bomb, which used a plutonium core.
58. Which among the following statement is correct
- Hydrogen bomb is based on the principle of nuclear fusion. A hydrogen bomb is always designed to have an inbuilt atom bomb which creates the high temperature and pressure required for fusion when it explodes.
- Then, fusion takes place in the hydrogen core and leads to the release of a very large amount of energy in an uncontrolled manner. The energy released in a hydrogen bomb (or fusion bomb) is much higher than that released in an atom bomb (or fission bomb).
- Only 1
- Only 2
- Both 1 and 2
- None
59. Which among the following is not the property of Nuclear Fission?
- The process of breaking up (splitting) of a heavy nucleus into two smaller nuclei is called ‘nuclear fission’.
- Extremely high temperature and pressure is needed.
- Alpha, beta and gamma radiations are emitted.
- Fission leads to emission of gamma radiation. This triggers the mutation in the human gene and causes genetic transform diseases.
Explanation
Nuclear Fission Can be performed at room temperature.
60. In 1932, who discovered the chargeless particles called neutrons?
- Goldstein
- Rutherford
- John Dalton
- James Chadwick
Explanation
In 1932, James Chadwick discovered the chargeless particles called neutrons. Presently, a large number of elementary particles like photon, meson, positron and neutrino have been discovered.
61. In 1911, which British scientist explained that the mass of an atom is concentrated in its central part called Nucleus?
- John Dalton
- J J Thomson
- Rutherford
- Goldstein
Explanation
In 1911, the British scientist, Ernest Rutherford explained that the mass of an atom is concentrated in its central part called Nucleus.
62. Which among the following is not the property of Nuclear Fusion?
- Nuclear fusion is the combination of two lighter nuclei to form a heavier nucleus.
- Extremely high temperature and pressure is needed.
- Alpha rays, positrons, and neutrinos are emitted.
- Only heat energy is emitted. Light energy is restricted in this.
Explanation
In Nuclear Fusion Only light and heat energy is emitted.
63. Which among the following statement is incorrect
- Sun fuses about 620 million metric tons of hydrogen each second and radiates about 3.8 × 1026 joule of energy per second.
- When this energy is radiated towards the Earth, it decreases in its intensity. When it reaches the Earth, its value is about 1.4 kilo joule per unit area in unit time.
- Only 1
- Only 2
- Both 1 and 2
- None
64. Which among the following radio isotope helps to increase the productivity of crops?
- Iodine
- Phosphorous
- Sodium
- Iron
Explanation
The radio isotope of phosphorous (P-32) helps to increase the productivity of crops. The radiations from the radio isotopes can be used to kill the insects and parasites and prevent the wastage of agricultural products. Certain perishable cereals exposed to radiations remain fresh beyond their normal life, enhancing the storage time. Very small doses of radiation prevent sprouting and spoilage of onions, potatoes and gram.
65. Which radio isotope is used for the effective functioning of heart?
- Radio iron
- Radio Iodine
- Radio Phosphorous
- Radio Sodium
Explanation
Radio sodium (Na24) is used for the effective functioning of heart.
66. Medical applications of radio isotopes can be divided into how many parts?
- Two
- Three
- Four
- Five
Explanation
Medical applications of radio isotopes can be divided into two parts: i) Diagnosis ii) Therapy. Radio isotopes are used as tracers to diagnose the nature of circulatory disorders of blood, defects of bone metabolism, to locate tumours, etc. Some of the radio isotopes which are used as tracers are: hydrogen, carbon, nitrogen, sulphur, etc.
67. Match the following radio isotopes with its respective medical purpose
- Radio – Iodine – 1. used in the treatment of skin diseases.
- Radio-iron – 2. used to cure goitre.
- Radio phosphorous – 3. are used in the treatment of skin cancer.
- Radio cobalt – 4. used to diagnose anaemia.
- 4 – 2 – 3 – 1
- 2 – 4 – 1 – 3
- 3 – 1 – 2 – 4
- 4 – 1 – 2 – 3
Explanation
Radio – Iodine (I131) is used to cure goitre. Radio-iron is (Fe59) is used to diagnose anaemia and also to provide treatment for the same. Radio phosphorous (P32) is used in the treatment of skin diseases. Radio cobalt (Co60) and radio-gold (Au198) are used in the treatment of skin cancer. Radiations are used to sterilize the surgical devices as they can kill the germs and microbes.
68. Which isotope is used in the airlines to detect the explosives in the luggage?
- Californium
- Americium
- Nobelium
- Dubnium
Explanation
An isotope of californium (Cf 252) is used in the airlines to detect the explosives in the luggage.
69. Which among the following statement is correct
- In industries, radioactive isotopes are used as tracers to detect any manufacturing defects such as cracks and leaks. Packaging faults can also be identified through radio activity. Gauges, which have radioactive sources are used in many industries to check the level of gases, liquids and solids
- Using the technique of radio carbon dating, the age of the Earth, fossils, old paintings and monuments can be determined. In radio carbon dating, the existing amount of radio carbon is determined and this gives an estimate about the age of these things.
- Only 1
- Only 2
- Both 1 and 2
- None
70. Which isotope is used in many industries as a smoke detector?
- Americium
- Fermium
- Nihonium
- Curium
Explanation
An isotope of Americium (Am241) is used in many industries as a smoke detector.
71. Identify A, B, C, and D from the following nuclear reactions.
- 13Al27 + A ——–> 15P30 + B
- 12Mg24 + B ——–> 11Na24 + C
- 92U238 + B ——–> 93Np239 + D
- A = 0n1; B = 2He4; C = –1e0; D = 1 H1
- A = 2He4; B= 0n1; C = –1e0; D = 1 H1
- A = 2He4; B= 0n1; C = 1 H1; D = –1e0
- A = 0n1; B = 2He4; C = 1 H1; D = –1e0
Explanation
13Al27 + 2He4——–> 15P30 + 0n1
12Mg24 + 0n1 ——–> 11Na24 + 1 H1
92U238 + 0n1 ——–> 93Np239 + –1e0
72. Which among the following statement is correct
- In day-to-day life, you do receive some natural radiation from the Sun. The radioactive elements present in the soil and rocks, the house hold appliances like television, microwave ovens, cell phones and the X-rays used in hospitals. These radiations do not produce any severe effects as they are very low in intensity.
- The second source of radiation exposure is man-made. These are due to nuclear reactors and during the testing of the nuclear devices in the atmosphere or in the ground. Improper and careless handling of radioactive materials release harmful radiations in our environment.
- These radiations are very harmful to the human body. A person who is exposed to radiations very closely or for a longer duration, is at a greater health risk and can be affected genetically.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
73. Which among the following organisation has recommended certain maximum permissible exposure limits to radiation that is believed to be safe without producing any appreciable injury to a person?
- ICRP
- IMF
- WANO
- IPSC
Explanation
The International Commission on Radiological Protection (ICRP) has recommended certain maximum permissible exposure limits to radiation that is believed to be safe without producing any appreciable injury to a person
74. The Safe limit of overall exposure to radiation is given as ____
- 20 milli sievert per year
- 30 milli sievert per year
- 40 milli sievert per year
- 60 milli sievert per year
Explanation
According to International Commission on Radiological Protection (ICRP) the Safe limit of overall exposure to radiation is given as 20 milli sievert per year. In terms of roentgen, the safe limit of receiving the radiation is about 100 m R per week.
75. Which is a device used to detect the levels of exposure to an ionizing radiation?
- Anemometer
- Refractometer
- Dosimeter
- Seismograph
Explanation
Dosimeter is a device used to detect the levels of exposure to an ionizing radiation. It is frequently used in the environments where exposure to radiation may occur such as nuclear power plants and medical imaging facilities. Pocket dosimeter is used to provide the wearer with an immediate reading of his/her exposure to X-rays and γ rays.
76. Which among the following is not the preventive measure for nuclear radiation?
- Radioactive materials should be kept in a thick walled lead container
- Iron coated aprons and Iron gloves should be used while working with hazardous radioactive materials.
- The radioactive materials should be handled only by tongs or by a remote-control device.
- Dosimeters should be worn by the users to check the level of radiation.
Explanation
Lead coated aprons and lead gloves should be used while working with hazardous radioactive materials. You should avoid eating while handling radioactive materials.
77. In which country the first nuclear reactor was built in 1942?
- Russia
- German
- USA
- Japan
Explanation
A Nuclear reactor is a device in which the nuclear fission reaction takes place in a self-sustained and controlled manner to produce electricity. The first nuclear reactor was built in 1942 at Chicago, USA.
78. A radon specimen emits radiation of 3.7 × 103 G Bq per second. Convert this disintegration in terms of curie. (one curie = 3.7 × 1010 disintegration per second)?
- 50 Curie
- 100 Curie
- 200 Curie
- 300 Curie
Explanation
1 Bq = one disintegration per second
one curie = 3.7 × 1010 Bq
1 Bq =
∴ 3.7 × 103 G Bq = 3.7 × 103 × 10 9 ×
= 100 Curie.
79. Which among the following is the type of Nuclear reactor?
- Breeder reactor
- Thermal reactor
- Gas-cooled reactor
- All the above
Explanation
Breeder reactor, fast breeder reactor, pressurized water reactor, pressurized heavy water reactor, boiling water reactor, water cooled reactor, gas-cooled reactor, fusion reactor and thermal reactor are some types of nuclear reactors, which are used in different places world-wide.
80. Which among the following in not the essential components of nuclear reactor?
- Moderator
- Control rod
- Coolant
- Cam shaft
Explanation
The essential components of a nuclear reactor are (i) fuel, (ii) moderator, (iii) control rod, (iv) coolant and (v) protection wall.
81. 92U235 experiences one α – decay and one β – decay. Find number of neutrons in the final daughter nucleus that is formed?
- Number of neutrons = 100
- Number of neutrons = 120
- Number of neutrons = 140
- Number of neutrons = 160
Explanation
Let X and Y be the resulting nucleus after the emission of the alpha and beta particles respectively.
92 U235 90X231 + 2He4
0X23191Y231 + -1e0
Number of neutrons = Mass number – Atomic number
= 231 – 91 = 140.
82. Which is used to slow down the high energy neutrons to provide slow neutrons?
- Coolant
- Moderator
- Control rod
- Dynamo
Explanation
A moderator is used to slow down the high energy neutrons to provide slow neutrons.
83. Which is commonly used fuel material in Nuclear reactor?
- Uranium
- Thorium
- Plutonium
- Curium
Explanation
A fissile material is used as the fuel. The commonly used fuel material is uranium.
84. Which among the following is the commonly used moderators?
- Lead
- Cesium
- Graphite
- Cadmium
Explanation
Graphite and heavy water are the commonly used moderators.
85. Which among the following statement is correct
- Control rods are used to control the number of neutrons in order to have sustained chain reaction. Mostly Xenon or Arsenic rods are used as control rods. They absorb the neutrons.
- A coolant is used to remove the heat produced in the reactor core, to produce steam. This steam is used to run a turbine in order to produce electricity.
- A thick concrete lead wall is built around the nuclear reactor in order to prevent the harmful radiations from escaping into the environment.
- Both 1 and 2
- Both 1 and 3
- Both 2 and 3
- All 1, 2 and 3
Explanation
Control rods are used to control the number of neutrons in order to have sustained chain reaction. Mostly boron or cadmium rods are used as control rods. They absorb the neutrons.
86. Which among the following is not the coolant?
- Water
- Air
- Helium
- None of the above
Explanation
Water, air and helium are some of the coolants. Nuclear reactors are widely used in power generation. They are also used to produce radio isotopes, which are used in a variety of applications. Some reactors help us to do research in the field of nuclear physics.
87. Which are used to convert non-fissionable materials into fissionable materials?
- Gas-cooled reactor
- Breeder Reactor
- Thermal reactor
- Water cooled reactor
Explanation
Breeder reactors are used to convert non-fissionable materials into fissionable materials.
88. Calculate the amount of energy released when a radioactive substance undergoes fusion and results in a mass defect of 2 kg?
- 1.8 × 1017 J
- 2.3 × 1021 J
- 1.8 × 1021 J
- 2.3 × 1017 J
Explanation
Mass defect in the reaction (m) = 2 kg
Velocity of light (c) = 3 × 108 m s-1
By Einstein’s equation, Energy released E = mc2
So, E = 2 × (3 × 108) 2 = 1.8 × 1017 J.
89. When Indian Atomic Energy Commission (AEC) was established?
- 1935
- 1945
- 1948
- 1952
Explanation
Indian Atomic Energy Commission (AEC) was established in August 1948 by the Department of Indian Scientific Research committee.
90. Who was the first chairman of Indian Atomic Energy Commission?
- Raja Raman
- Homi Jahangir Bhabha
- Vikram Sarabhai
- Satyendra Nath Bose
Explanation
Dr. Homi Jahangir Bhabha was the first chairman of Indian Atomic Energy Commission. Now, it is known as Bhabha Atomic Research Centre (BARC).
91. Which is India’s first nuclear power station?
- Kaiga Atomic Power Station
- Kalpakkam Atomic Power Station
- Narora Atomic Power Station
- Tarapur Atomic Power Station
Explanation
Nuclear power is the fifth largest source of power in India. Tarapur Atomic Power Station is India’s first nuclear power station.
92. Which among the following state has two atomic power station?
- Maharashtra
- Tamil Nadu
- Rajasthan
- Gujarat
Explanation
Tamil Nadu is the only state with two atomic power station in Kalpakkam and Kudankulam
93. How many power stations are there now in India?
- Three
- Four
- Seven
- Eight
Explanation
Now, there are a total of seven power stations, one each in Maharashtra, Rajasthan, Gujarat, Uttar Pradesh and two in Tamil Nadu. In Tamil Nadu, we have nuclear power stations in Kalpakkam and Kudankulam.
94. Which was the first nuclear reactor built in India and Asia?
- Apsara
- Cirus
- Purnima
- Dhuruva
Explanation
Apsara was the first nuclear reactor built in India and Asia.
95. How many nuclear reactors are now operating in India?
- 12
- 18
- 22
- 28
Explanation
Now, there are 22 nuclear reactors which are operating in India.
96. Which among the following is operating reactors in India?
- Cirus
- Dhuruva
- Purnima
- All the above
Explanation
Some operating reactors in India are 1. Cirus, 2. Dhuruva and 3. Purnima.
97. Where Indian Atomic Energy Commission (AEC) was established in August 1948?
- Maharashtra
- Gujarat
- West Bengal
- Tamil Nadu
Explanation
Indian Atomic Energy Commission (AEC) was established in August 1948 at Bombay (now Mumbai) in Maharashtra. It is the nodal agency for all the research done in the field of atomic energy.
10th Science Lesson 7 Questions in English
7] Atoms And Molecules
1. Assertion (A): The Greek philosophers first proposed the idea of atom in fifth century BCE.
Reasoning(R): The Greek theory of atom was more philosophical than scientific.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
Matter is made of atoms. Curiously the idea of atom was first proposed by the Greek philosophers in the fifth century BC (BCE). But their theory was more philosophical than scientific.
2. Who proposed the first scientific theory of atom?
a) John Dalton
b) Ernest Rutherford
c) Robert Boyle
d) William Ramsay
Explanation
The first scientific theory of the atom was proposed by John Dalton.
3. Which of this scientist did not agree with Dalton’s theory?
a) J.J. Thomson
b) Rutherford
c) Neil’s Bohr
d) All the above
Explanation
Few of the postulates of Dalton’s theory about an atom were found incorrect by the later on studies made by J.J. Thomson, Rutherford, Neil’s Bohr and Schrodinger.
4. Assertion (A): All the Atoms are indivisible.
Reasoning(R): An atom is made up of protons, neutrons and electrons.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is False but R is True.
d) Both A and R is False.
Explanation
‘The main postulates of modern atomic theory’ are as follows: An atom is no longer indivisible (after the discovery of the electron, proton, and neutron).
5. Define Isobar.
a) Atoms of same element and same atomic mass.
b) Atoms of different element and different atomic mass.
c) Atoms of same element and different atomic mass.
d) Atoms of different element and same atomic mass.
Explanation
Atoms of the same element may have different atomic mass. (Discovery of isotopes 17Cl35, 17Cl37). Atoms of different elements may have same atomic masses (discovery of Isobars 18Ar40, 20Ca40).
6. Which of this concept describe the indestructible nature of an atom?
a) Isotopes
b) Artificial Transmutation
c) Isomer
d) Isobar
Explanation
Atoms of one element can be transmuted into atoms of other elements. In other words, atom is no longer indestructible (discovery of artificial transmutation).
7. What is the chemical composition of the natural honey?
a) C6H12 O6
b) C4H10O2
c) C12H20O6
d) C1H4O4
Explanation
Atoms may not always combine in a simple whole number ratio (E.g. Glucose C6H12O6 C:H:O = 6:12:6 or 1:2:1 and Sucrose C12H22O11 C:H:O = 12:22:11).
8. In which of this smallest particle a chemical reaction occurs?
a) Atoms
b) Molecules
c) Protons
d) Molar
Explanation
Atom is the smallest particle that takes part in a chemical reaction. The mass of an atom can be converted into energy (E = mc2).
9. Which of the following is not a subatomic particle?
a) Proton
b) Neutron
c) Molecule
d) Electron
Explanation
Atoms are the building blocks of matter. Since matter has mass, it must be due to its atoms. According to the modern atomic theory, an atom contains subatomic particles such as protons, neutrons and electrons.
10. What is defined as mass number of an atom?
a) Sum of number of protons and neutrons
b) Sum of number of neutrons
c) Sum of number of electrons and protons
d) Sum of number of electrons
Explanation
Protons and neutrons have considerable mass, but electrons don’t have such a considerable mass. Thus, the mass of an atom is mainly contributed by its protons and neutrons and hence the sum of the number of protons and neutrons of an atom is called its mass number.
11. Which of these is used to measure the mass of an atom?
a) Mass number
b) Milligram
c) Atomic mass unit
d) Nanometer
Explanation
Individual atoms are very small and it is difficult to measure their masses. You can measure the mass of macroscopic materials in gram or kilogram. The mass of an atom is measured in atomic mass unit (amu).
12. Which of the following is not true regarding the atomic mass unit?
a) One-twelfth of mass of a Carbon-12 atom.
b) Carbon-12 is an isobar of carbon.
c) Carbon-12 contains 6 protons and 6 neutrons.
d) Carbon-12 is an isotope of carbon.
Explanation
Atomic mass unit is one-twelfth of the mass of a carbon-12 atom; an isotope of carbon, which contains 6 protons and 6 neutrons.
13. Which of this symbol is used to denote the unified atomic mass in the modern system?
a) A
b) U
c) M
d) Au
Explanation
The symbol ‘amu’ is no longer used in the modern system and instead it uses the symbol ‘U’ to denote unified atomic mass.
14. What is the approximate mass of a proton or neutron?
a) 10 amu
b) 1 amu
c) 100 amu
d) 0.01 amu
Explanation
The mass of a proton or neutron is approximately 1 amu.
15. Assertion (A): Relative mass was used to measure the atomic mass by elements of equal number of atoms.
Reasoning(R): The absolute mass of an atom cannot be determined directly.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
As an atom is very small, its absolute mass cannot be determined directly. The early pioneers of chemistry used to measure the atomic mass of an atom relative to an atom of another element. They measured the masses of equal number of atoms of two or more elements at a time, to determine their relative masses.
16. Which of these is used as a standard for measuring atomic mass?
a) A standard element
b) Planks constant
c) Atmospheric pressure
d) Standard temperature
Explanation
The established one element as a standard, gave it an arbitrary value of atomic mass and using this value they measured the relative mass of other elements. The mass obtained by this way is called relative atomic mass.
17. Which of the atom is not a standard mass for relative atomic mass?
a) Hydrogen
b) Oxygen
c) Carbon
d) Helium
Explanation
The mass of hydrogen atom was chosen as a standard and masses of other atoms were compared with it because of the existence of isotopic character of hydrogen (1H1, 1H2, 1H3).
18. Which of these is not an isotope of hydrogen atom?
a) 1H2
b) 1H1
c) 1H8
d) 1H3
Explanation
The mass of hydrogen atom was chosen as a standard and masses of other atoms were compared with it because of the existence of isotopic character of hydrogen (1H1, 1H2, 1H3).
19. Which of this carbon isotope is used as standard measure for relative atomic mass?
a) C-17
b) C-19
c) C-12
d) C-15
Explanation
Later hydrogen atom was replaced by oxygen atom as the standard. Now the stable isotope of carbon (C-12) with atomic mass 12 is used as the standard for measuring the relative atomic mass of an element.
20. Choose the Incorrect statements regarding relative atomic mass.
i) 1/10 th part of mass of a carbon-12 atom.
ii) Denoted by Ar.
iii) Also known as Standard Atomic Weight.
a) i only
b) ii only
c) iii only
d) None of the above
Explanation
Relative atomic mass of an element is the ratio between the average mass of its isotopes to 1/ 12th part of the mass of a carbon-12 atom. It is denoted as Ar. It is otherwise called “Standard Atomic Weight”.
21. What is defined as relative atomic mass?
a) Ratio between Average mass of isotopes and mass of carbon atom.
b) Ratio of Total number of isotopes of element to the mass of carbon.
c) Average mass of carbon atoms
d) Ratio of Average mass of isotopes of the element and 1/12th of mass of carbon-12 atom.
Explanation
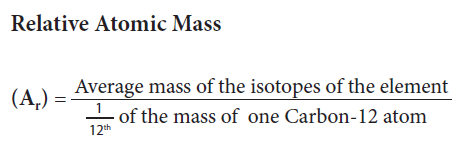
22. State the modern method of determining atomic mass?
a) Vernier caliper
b) Mass Spectrometry
c) Physical Balance
d) Standard Elements
Explanation
Modern methods of determination of atomic mass are done by Mass Spectrometry which uses C-12 as standard.
23. Which of the given element has relative atomic mass value as one?
a) Hydrogen
b) Sulphur
c) Sodium
d) Nitrogen
24. Identify the incorrect match.
A. O i) 16
B. Mg ii) 24
C. N iii) 10
D. C iv) 12
a) i only
b) ii only
c) iii only
d) iv only
Explanation
Relative atomic mass of elements (C-12 Scale)
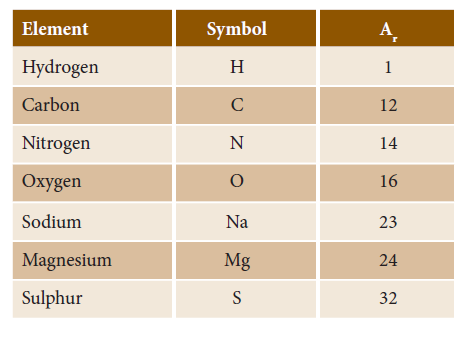
25. What is the unit of relative atomic mass?
a) No unit
b) Grams
c) amu
d) None of the above
Explanation
Relative Atomic Mass is only a ratio so it has no unit. If the atomic mass of an element is expressed in grams it is called as Gram Atomic Mass.
26. What is the gram atomic mass of nitrogen?
a) 1g
b) 16g
c) 12g
d) 14g
Explanation
Gram Atomic Mass of hydrogen = 1 g
Gram Atomic Mass of carbon = 12 g
Gram Atomic Mass of nitrogen = 14 g
Gram Atomic Mass of oxygen = 16 g
27. Assertion (A): The isotopic mixture of elements is considered to calculate the atomic mass of an element.
Reasoning(R): Naturally occurring element exists as a mixture of isotopes with their own mass.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
It is somewhat more complicated because most of the naturally occurring elements exist as a mixture of isotopes each of which has its own mass. Thus it is essential to consider this isotopic mixture while calculating the atomic mass of an element.
28. Which of the following is used to measure the average atomic mass of an element?
a) Natural isotopes
b) Chemical reactions
c) Available compounds
d) All the above
Explanation
The average atomic mass of an element is the weighted average of the masses of its naturally occurring isotopes.
29. Calculate the average atomic mass of an element having 2 isotopes of 75% and 25% with masses 10, 20 respectively?
a) 25
b) 10.5
c) 100
d) 12.5
Explanation
Average atomic mass = (Mass of 1st isotope × % abundance of 1st isotope) + (Mass of 2nd isotope ×% abundance of 2nd isotope)
Thus for the given element the average atomic mass = (10 × 75 / 100) + (20 × 25 / 100) = 12.5
30. Choose the correct statements.
i) Atomic weight is also used as Average atomic mass.
ii) Atomic mass of all the elements is whole numbers.
a) i only
b) ii only
c) Both i and ii
d) Neither i nor ii
Explanation
The atomic masses of elements given in the periodic table are average atomic masses. Sometimes the term atomic weight is used to mean average atomic mass. It is observed from the periodic table that atomic masses of most of the elements are not whole numbers.
31. Which of these carbon isotopes are considered for calculating the atomic mass of carbon?
a) C-12, C-9
b) C-11, C-7
c) C-12, C-13
d) C-5, C-17
Explanation
To calculate the atomic mass of carbon both of its natural isotopes such as carbon-12 and carbon-13 are considered. The natural abundance of C-12 and C-13 are 98.90 % and 1.10 % respectively. The average of the atomic mass of carbon is calculated as follows:
Average atomic mass of carbon = (12 × 9 8 . 9 ) 100 + (13 × ) 1.1 100
32. Match the atomic mass and its elements.
A. Lithium i) 1.008
B. Hydrogen ii) 4.003
C. Boron iii) 6.941
D. Helium iv) 10.811
a) ii, iv, i, iii
b) iii, i, iv, ii
c) ii, iii, i, iv
d) iv, i, iii, ii
Explanation
Atomic mass of some elements
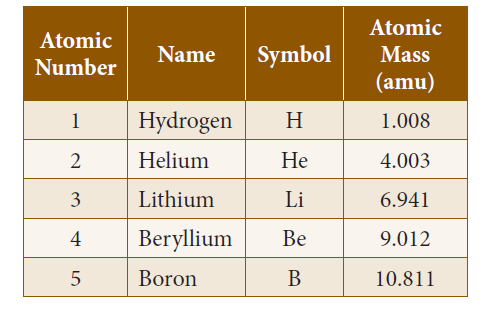
33. Which of this Oxygen isotope is abundantly available in nature?
a) 8O16
b) 5O11
c) 8O17
d) 8O15
Explanation
Isotopes of oxygen

34. Which of this element is not found as single atom by nature?
a) Helium
b) Argon
c) Fluorine
d) Xenon
Explanation
Except noble gases atoms of most of the elements are found in the combined form with itself or atoms of other elements. It is called as a molecule.
35. Define a molecule.
a) Combination of two atoms only.
b) Physical attraction of atoms.
c) Combination of two or more atoms.
d) None of the above.
Explanation
A molecule is a combination of two or more atoms held together by strong chemical forces of attraction, i.e. chemical bonds.
36. Assertion (A): A molecule may be an element or a compound.
Reasoning(R): Molecules may contain atoms of same element or two or more elements.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
A molecule may contain atoms of the same element or may contain atoms of two or more elements joined in a fixed ratio, in accordance with the law of definite proportions. Thus, a molecule may be an element or a compound.
37. Which of the following denotes a homoatomic molecule?
a) Similar kind of atoms
b) Single atom element
c) Simple chemical reaction
d) Similar atomic value
Explanation
If the molecule is made of similar kind of atoms, then it is called homoatomic molecule.
38. Assertion (A): Heteroatomic molecule consists of atoms of different elements.
Reasoning(R): A compound is also referred as a heteroatomic molecule.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
The molecule that consists of atoms of different elements is called heteroatomic molecule. A compound is a heteroatomic molecule.
39. What is referred as atomicity?
a) Total number of molecules
b) Number of free electrons
c) Number of atoms in a molecule
d) Number of chemical forces connecting atoms.
Explanation
The number of atoms present in the molecule is called its ‘atomicity’.
40. How many number of atoms are present in polyatomic elements?
a) =3
b) <3
c) > 3
d) All the above
Explanation
Classification of molecules
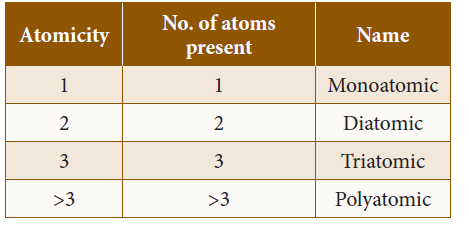
41. Ozone,
i) Has three oxygen molecules.
ii) It is a homotriatomic molecule.
iii) It is also called as polyatomic molecule.
a) i only
b) ii only
c) iii only
d) All the above
Explanation
Ozone (O3) contains three oxygen atoms and hence it is called homotriatomic molecule. If a molecule contains more than three atoms, then it is called polyatomic molecule.
42. A Heterodiatomic molecule,
a) Consists of two atoms
b) Consists of same element atoms
c) Atomicity value is one.
d) Two atoms of different elements.
Explanation
Consider hydrogen chloride. It consists of two atoms, but of different elements, i.e. hydrogen and chlorine. So, its atomicity is two. It is a heterodiatomic molecule.
43. What is the atomicity value of the water molecule?
a) 2
b) 3
c) 4
d) 1
Explanation
The water molecule contains two hydrogen atoms and one oxygen atom. So its atomicity is three. It is a heterotriatomic molecule.
44. Which of this carbon isotope is used to calculate the relative molecular mass of a molecule?
a) C-12
b) C-10
c) C-9
d) C-17
Explanation
The Relative Molecular Mass of a molecule is the ratio between the mass of one molecule of the substance to 1 /12th mass of an atom of Carbon -12.
45. What is the relative molecular mass of water?
a) 18
b) 12
c) 10
d) 3
Explanation
Relative molecular mass of water
(H2O) is calculated as follows: A water molecule is made of 2 atoms of hydrogen and one atom of oxygen. So, the relative molecular mass of water
= (2 × mass of hydrogen) + (1 × mass of oxygen)
= (2 × 1) + (1 × 16)
= 18 i.e., one molecule of H2O is 18 times as heavy as 112th of the mass of a carbon –12.
46. Assertion (A): Molecular mass of a compound in grams is called Gram molecular mass.
Reasoning(R): Relative Molecular mass is a ratio value expressed in grams.
a) Both A and R is True and R is the correct explanation of A.
b) Both A and R is True but R is not the correct explanation of A.
c) A is True but R is False.
d) Both A and R is False.
Explanation
Relative Molecular Mass is only a ratio. So, it has no unit. If the molecular mass of a compound is expressed in grams, it is called Gram Molecular Mass.
47. What is the gram molecular mass value of carbon dioxide?
a) 17g
b) 36.5g
c) 4.4g
d) 44g
Explanation
Gram Molecular Mass of water = 18 g
Gram Molecular Mass of carbon dioxide = 44 g
Gram Molecular Mass of ammonia = 17 g
Gram Molecular Mass of HCl = 36.5 g
48. Choose the correct statements regarding atoms.
i) Atom is the smallest particle of an element.
ii) Atoms have a chemical bond.
iii) All the atoms are highly reactive.
a) i only
b) ii only
c) iii only
d) All the above
Explanation

49. Which of these exist in a free state?
a) Atom
b) Neutron
c) Molecule
d) Proton
50. Which of this statement is not correct?
a) Noble gas atoms exist in Free State.
b) Molecules are highly reactive.
c) Molecule is the smallest particle of a compound.
d) Atoms in a molecule are held by chemical bonds.
Explanation

51. In which of this scale the atomic mass is measured?
a) U
b) Milligrams
c) Relative scale
d) A
Explanation
Atomic mass units provide a relative scale for the masses of the elements. Since the atoms have such small masses, no usable scale can be devised to weigh them in the calibrated units of atomic mass units.
52. Which of these is used to denote the number of particles?
a) Mass
b) Mole
c) Numeric
d) Grams
Explanation
Chemists measure atoms and molecules in ‘moles’. So you can now understand that ‘mole’ denotes a number of particles.
53. Which of these entities are included in SI system calculation for a mol?
a) Atoms
b) Molecules
c) Ions
d) All the above
Explanation
In the SI system, the mole (mol) is the amount of a substance that contains as many elementary entities (atoms, molecules, or other particles) as there are atoms in exactly 12 g (or 0.012 kg) of the carbon-12 isotope.
54. Which of these represents the actual number of atoms in 12g of carbon-12?
a) Avogadro Number
b) Atomic Mass Number
c) Atomic Molecule Number
d) Molecule Number
Explanation
The actual number of atoms in 12 g of carbon-12 is determined experimentally. This is called Avogadro’s number (NA)
55. What is the value of Avogadro Number?
a) 6.674 *10-11
b) 6.626 *10-34
c) 6.023 * 1023
d) 5.6703* 10-8
Explanation
NA is named after an Italian scientist Amedeo Avogadro who proposed its significance. Its value is 6.023 × 1023. So one mole of a substance contains 6.023 × 1023 entities.
56. Which of these values is counted in moles?
a) Mass
b) Particles
c) Atoms
d) Molecules
Explanation
Mole Concept: The study of the collection of particles by using mole as the counting unit in order to express the mass and volume of such unit particles in a bulk of matter is known as mole concept.
57. Which of these data’s can be counted by the number of moles?
a) Number of moles of molecules.
b) Number of moles in a chemical reaction.
c) Number of moles of ions.
d) Number of moles of atoms.
Explanation
The number of moles of a substance can be calculated by various means depending on the data available, as follows: Number of moles of molecules. Number of moles of atoms. Number of moles of a gas (Standard molar volume at STP = 22.4 litre). Number of moles of ions.
58. What is the standard pressure value of a STP?
a) 1.00 atm
b) 10 atm
c) 0.001 atm
d) 100 atm
Explanation
STP-Standard Temperature and Pressure (273.15 K,1.00 atm)
59. Which of these given values are equal to one mole of an element?
a) Gram atomic mass
b) Relative atomic mass
c) Average atomic mass
d) Total number of isotopes
Explanation
One mole of an element contains 6.023 × 1023 atoms and it is equal to its gram atomic mass .i.e., one mole of oxygen atom contains 6.023 × 1023 atoms of oxygen and its gram atomic mass is 16 g.
60. What is the value of one mole of a matter?
a) 6.023 * 1023
b) Avogadro Number
c) NA
d) All the above
Explanation
One mole of matter contains 6.023 × 1023 molecules and it is equal to its gram molecular mass.
61. How much volume does one mole of gas occupies at STP?
a) 22.4 liter
b) 220 ml
c) 224000 liter
d) 2240 ml
Explanation
One mole of any gas occupies 22.4 liter or 22400 ml at S.T.P. This volume is called as molar volume.
62. Which of the following is not correct regarding the number of moles calculation?
a) Mass / Average number of isotopes
b) Mass / Molecular mass
c) Number of atoms / 6.0238* 1023
d) Mass / Atomic mass
Explanation
Calculation of number of moles by Different modes
Number of moles = Mass / Atomic Mass
= Mass / Molecular mass
= Number of Atoms / 6.023 × 1023
= Number of Molecules / 6.023 × 1023
63. The percentage composition represents the ____ of each element in ___ of the compound.
a) Number of molecules, 10 g
b) Number of atoms, 100g
c) Mass, 100g
d) Average mass, 10g
Explanation
The percentage composition of a compound represents the mass of each element present in 100 g of the compound.
64. What is the value of the mass percentage of an element?
a) Mass of element in compound / molecular mass of the compound * 100
b) Average mass value of compound / atomic mass of the element * 100
c) Mass of the compound / molecular mass of the compound * 100
d) Atomic mass value of compound / molecular value of isotopes * 100
Explanation
Mass % of an element = mass of that element in the compound / molecular mass of the compound * 100
Now, molecular mass of H2O = 2(1) + 16 = 18 g
Mass % of hydrogen = 2 × 100 18 = 11.11 %
Mass % of oxygen = 16 × 100 18 = 88.89 %
This percentage composition is useful to determine the empirical formula and molecular.
65. When did Avogadro frame a hypothesis relating volume of gas and molecules?
a) 1811
b) 1732
c) 1891
d) 1632
Explanation
In 1811 Avogadro framed a hypothesis based on the relationship between the numbers of molecules present in equal volumes of gases in different conditions.
66. Define the Avogadro’s law?
a) Equal volume of all gas varies with unequal number of molecules.
b) Equal volume of all gas contains equal number of molecules under ideal condition.
c) Unequal volume of all gas contains equal number of molecules.
d) Under similar conditions of temperature and pressure equal volume of all gas contains equal number of molecules.
Explanation
The Avogadro’s law states that “equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules”
67. Which of the following represents the Avogadro law?
a) V = n
b) V α n
c) V < n
d) V > n
Explanation
It follows that the volume of any given gas must be proportional to the number of molecules in it. If ‘V’ is the volume and ‘n’ is the number of molecules of a gas, then Avogadro law is represented, mathematically, as follows: V α n
V = constant × n
68. How many oxygen atoms are in Potassium permanganate?
a) 3
b) 4
c) 1
d) 5
Explanation
The chemical formula for potassium permanganate is KMnO4 which has one potassium, one manganese and four oxygen atoms.
69. Which of these is not an application of Avogadro’s law?
a) Determining the atomicity of gas.
b) Determining the average mass of all gases.
c) Relation between molecular mass and vapor density.
d) Determining gram molar volume of gas.
Explanation
APPLICATIONS OF AVOGADRO’S LAW: It explains Gay-Lussac’s law. It helps in the determination of atomicity of gases. Molecular formula of gases can be derived using Avogadro’s law. It determines the relation between molecular mass and vapor density. It helps to determine gram molar volume of all gases (i.e, 22.4 liter at S.T.P)
70. Which of these are not related in calculating the relative molecular mass by hydrogen scale?
a) Relative Molecular Mass of a gas
b) Mass of one molecule of a gas
c) Mass of one atom of Hydrogen
d) Average mass of isotopes
Explanation
Relative molecular mass: (Hydrogen scale) The Relative Molecular Mass of a gas or vapor is the ratio between the mass of one molecule of the gas or vapor to mass of one atom of Hydrogen.
71. What are the factors considered to measure the vapor density of a gas?
a) Temperature
b) Pressure
c) Viscosity
d) Both a and b
Explanation
Vapor density is the ratio of the mass of a certain volume of a gas or vapor to the mass of an equal volume of hydrogen measured under the same conditions of temperature and pressure. Vapor Density (V.D.) = Mass of a given volume of gas or vapor at S.T.P. / Mass of the same volume of hydrogen
72. What is the vapor density value at STP?
a) Mass of total molecules / mass of hydrogen
b) Mass of n molecules of gas / Mass of n molecules of hydrogen
c) Average molecular value of gas / Mass of hydrogen isotopes
d) Total number of isotopes / Average number of isotopes of hydrogen
Explanation
V.D. at S.T.P = Mass of ‘n’ molecules of a gas or vapor at S.T.P. / Mass of ‘n’ molecules of hydrogen
73. Which of the following is the value of Vapor density?
a) Relative molecular mass / 2
b) Relative molecular mass * 4
c) Relative molecular mass / 4
d) Relative molecular mass * 2
Explanation
Relative molecular mass = 2 × Vapor density
74. Calculate percentage of Hydrogen in H2SO4.
a) 20
b) 2.04
c) 32
d) 64
Explanation
Molecular mass of H2SO4 = (1 × 2) + (32 × 1) + (16 × 4)
= 2 + 32 + 64
= 98 g
% of H in H2SO4 = Mass of hydrogen / Molecular mass of H2SO4 * 100
% of H in H2SO4 = 2/ 98 * 100 = 2.04
75. Calculate the number of moles in 10 liter of CO2 at S.T.P.
a) 0.446
b) 0.23
c) 2.34
d) 4.4
Explanation
Number of moles of CO2 = Volume at S.T.P / Molar volume
= 10 / 22.4 = 0.446